- Journal List
- Lymphat Res Biol
- PMC3780287
Lymphatic Function and Immune Regulation in Health and Disease
 Corresponding author.
Corresponding author.Lymphatic vessels are present throughout the body, except in the central nervous system and bone marrow. The lymph nodes (LNs), spleen, and other secondary lymphoid organs (e.g., Peyer's patches) are strategically located in anatomical sites where they collect foreign antigen and antigen presenting cells to activate antigen-specific lymphocytes efficiently.1 In the peripheral tissues, specialized lymphatic capillaries—called initial lymphatic vessels—allow soluble materials and cells to enter the lymphatic system easily. The collected fluid and cells form lymph, which is transported by smooth muscle-invested collecting lymphatic vessels to the draining lymph node. The lymph node provides a highly organized microarchitecture that supports optimal immune surveillance. The “filtered” lymph fluid, as well as naïve and activated lymphocytes, exit the lymph node via efferent lymphatic vessels. Lymphatic capillaries, collecting lymphatic vessels, and lymph nodes together provide protective immunity for the body. Disruptions of lymphatic function compromise immune function and result in lymphedema. In this review, we will summarize the current knowledge of how lymphatic function is altered in inflammatory states, cancer, and infection.
Lymphatic Vessels
Blood capillaries consist of endothelial cells that are connected by intercellular tight junctions and surrounded by a continuous basement membrane and pericytes. In contrast, lymphatic capillaries are composed of endothelial cells supported by a thin, discontinuous basement membrane with sparse pericyte associations. Capillary lymphatic endothelial cells (LECs) are linked to each other with button-like intercellular junctions that make lymphatic capillaries highly permeable to interstitial fluid and its contents.2 These oak leaf-shaped LECs have a unique microarchitecture, in which overlapping flaps of adjacent cells form primary valve structures. These primary valve structures are critical for the formation of lymph. When tissue pressure increases above that in the lymphatic capillary, the primary valves open to allow lymph to enter freely into the lymphatic vessel.3,4 These primary valves also provide large enough gaps for dendritic cells (DCs) to enter the vessel without the need for integrin adhesion or pericellular proteolysis.5 Antigen presenting cells, mostly DCs, migrate to lymphatic capillaries using local chemokine CCL21 gradients produced by LECs and interstitial flow.6–9 When activated, DCs enter the lymphatic capillaries and interact with LECs while migrating towards the lymph node.10
Lymphatic capillaries merge into collecting lymphatic vessels. The LECs in collecting lymphatic vessels are tightly connected in a continuous “zipper-like” junction pattern.2 Collecting lymphatic vessels are invested in smooth muscle cells (SMCs) and have intraluminal lymphatic valves; the vessel segment between two such valves is known as a lymphangion. Intraluminal lymphatic valves maintain unidirectional lymph flow by preventing flow back toward the periphery. Smooth muscle cells are well organized along the lymphangion, but are scattered sparsely around the valve. As such, lymphangions are muscular pumping units that propel lymph through the lymphatic system. In physiological conditions, both active and passive forces drive lymph flow.11,12 Passive forces include arterial vasomotion, venous pressure, skeletal muscle motion, or drainage by gravity. In many circumstances, a more active mechanism—the autonomous SMC-mediated contractions of lymphatics vessels—is needed to drive lymph through lymph nodes toward the blood circulation.13–15 These inherent lymphatic contractions are vital components in the maintenance of both fluid homeostasis and the transport of lymphocytes. Numerous factors have been shown to regulate lymphatic contractility, including endothelial cell-derived nitric oxide (NO) and certain neurotransmitters.
NO is produced by three isoforms of nitric oxide synthase (NOS)—endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS). eNOS is expressed by LECs, but it is not expressed uniformly along collecting lymphatic vessels; instead, NO production is higher near the intraluminal valve relative to the tubular portions.16–19 During phasic pumping, NO is also produced at specific times,18,19 which is thought to be critical for sustained lymphatic contractions.20,21 Spatial and temporal gradients are created by the rapid decay of NO in vivo. During the systolic phase of a contraction, NO is released near the valves in a process that is hypothesized to be activated by lymph flow.18 NO, acting on the SMCs investing the collecting lymphatic vessel, triggers vessel opening and allows for lymphangion filling. The relaxation signal dissipates as NO decays, which leads to the start of the next contraction. eNOS blockade decreases lymph fluid velocity and increases the pumping frequency of mesenteric lymphatic vessels.14,16,22 In vivo, lymphatic contractions are much weaker in eNOS-/- mice, but there is a somewhat paradoxical increase in lymphatic diameter. This observation suggests other cellular, metabolic, and/or nervous system control pathways act in concert with NO on SMCs.14,23–25
The endothelium of collecting lymphatic vessels is generally thought to prevent transvascular cell or fluid transport. However, this notion of a passive endothelium may not be accurate. There is evidence that DCs interact with LECs in initial lymphatic vessels.10,26 Furthermore, there are many ongoing investigations trying to understand if the permeability of collecting lymphatic vessels may represent an additional avenue for antigen collection. Collecting lymphatic vessels have been difficult to study in vivo, which in turn has limited our understanding of their biology, molecular regulation, and function.
Lymph Nodes
Lymph transported through collecting lymphatic vessels is concentrated in the draining lymph node, which is the key location where adaptive immune responses are initiated. The unique lymph node microenvironment is organized to provide optimal conditions for naïve lymphocytes—which enter the lymph node through high endothelial venules (HEVs)—to interact with antigen and DCs for immune surveillance. The cell contents in the incoming afferent lymph are mostly memory T cells and DCs. The outgoing efferent lymph is composed of a greater number of B cells, together with T cells that have surveyed antigens in that particular lymph node. Myeloid cells usually turn over locally without exiting the lymph node.
Lymphatic vessels surround the lymph node, thereby forming the subcapsular and medullary sinuses. Lymphatic vessels are also found in the hilum, and facilitate the outflow of lymph from the lymph node. Macrophages are most often found in these lymphatic-rich areas. Blood vessels enter and exit the lymph node from the hilum. They run through the medulla, and they branch within and are distributed throughout the cortex. In the paracortex, blood vessels specialize into HEVs, which facilitate lymphocyte homing into LNs from the blood.27,28 The L-selectin expressing naïve lymphocytes tether with peripheral node addressin (PNAd) on HEVs, followed by chemokine activation and tight binding of integrins. This facilitates the diapedesis of lymphocytes for transendothelial migration.29–33 Lymph node stromal cells, which include endothelial cells, fibroblastic reticular cells (FRCs), follicular stromal cells, and marginal reticular cells, express chemokines to orchestrate cell compartmentalization. HEVs and fibroblastic reticular cells express CCL19 and CCL21 to attract CCR7-expressing T cells and DCs. Therefore, activated DCs and T cells are efficiently concentrated in the paracortical zone to facilitate antigen-specific T cell activation.34,35 Follicular stromal cells, follicular DCs, and marginal reticular cells each express CXCL13, which guides CXCR5-expressing B cell homing to B cell follicles located in the cortex of the lymph node. Sphingosine-1-phosphate, a lipid signaling molecule expressed—along with its receptor—by LECs, facilitates the egress of lymphocytes from LNs into efferent lymphatic vessels.36,37
Functional lymphatic vessels are required for the maintenance of the LN micro-architecture.11,38–40 Severing afferent lymphatic vessels produces dramatic effects in HEVs. These manifest as gross morphological changes, reduced lymphocyte adherence to the vessels,39–41 altered expression of several HEV genes,39,40,42 and a decrease in the uptake of 35S-sulfate (a functional marker of PNAd modification).43,44 Why lymphatic function is required to maintain the normal LN microenvironment is not clear, but several facts suggest an answer. The majority of HEVs are located in the paracortical area, which is separate from the lymphatic vessels. However, a reticular network within the lymph node known as the conduit system enables lymph factors to reach HEVs quickly.28,45,46 Low molecular weight materials (below 70 kD) move rapidly via the conduit system and reach the wall of HEVs within minutes. Lymph-borne chemokines likely adopt this route to regulate HEV function and rapidly change lymphocyte HEV transmigration.47–49 DCs and macrophages are also important in maintaining the normal HEV phenotype while regulating lymphocyte entry into the lymph node.50,51 These activities are each dependent on the entry of lymph into the node. Thus, when lymphatic function is disrupted, abnormal lymph node function would be predicted.
A critical element of immune function is the efficient interaction between activated APCs and naïve T cells. For this to occur, free antigen or the APCs themselves must localize to the T cell zone of the lymph node. Small, free antigen that enters the lymph node moves rapidly along the conduit system where it is taken up and processed by resident DCs.46–47 However, large antigens and microbial particles cannot penetrate deep into the LN via the conduit system. These particles enter the LN sinus and are sampled by macrophages and B cells.52–54 These lymphatic-associated macrophages prevent pathogens from exiting the lymph node without being properly screened.55 In addition to free antigen entering the node, DCs from the peripheral tissue can migrate to LNs and initiate T-cell responses. This mechanism occurs much more slowly than the presentation of free antigen by resident DCs.35
The stimulated tissue-migrating DCs have increased expression of co-stimulatory molecules and CCR7. CCL19 and CCL21 produced by HEVs and FRCs support CCR7-dependent migration of activated DCs towards the T cell zone in the draining LN.6,56 The migrating tissue DCs play a different role in initiating T cell responses than do resident DCs of the lymph node. However, both are required for priming CD4 T cells.57 Combining all of these mechanisms, the lymph node provides a unique “filter” structure to capture antigen or migrating DCs in the lymph node. The LN ultimately serves as a site for activation of lymphocytes that attack foreign antigens and restrict systemic spread of infections.
A fine-tuned immune response generates an immune reaction to foreign antigens while preventing overt reactions to self-antigens. Infection and inflammation cause extensive host cell damage and release large quantities of self-antigens. Not all self-antigens have access to the thymus during the development of the T cell repertoire. Thus, autoreactive T cells that escape from central tolerance need to be silenced in the periphery.58 During normal conditions, LNs function as a niche for generating peripheral tolerance as DCs continuously sample self-antigens prior to migrating to the draining lymph node.59–65 Most self-antigen-bearing DCs in lymph nodes are immature.66 These cells express low levels of costimulatory molecules and limit self-reactive T cell response by inducing anergy, clonal deletion, and/or the expansion of regulatory T cells.59,60,66–68 In addition, nonhematopoietic stromal cells in the lymph node (e.g., LECs, FRCs) also promote tolerance by presenting self-antigens and depleting self-reactive CD8 T cells via clonal deletion.69–71
Lymphatics in Inflammation
Inflammation causes lymphangiogenesis and expansion of the lymphatic network (Fig. 1). However, changes to lymphatic drainage to the lymph node during lymphangiogenesis appear to depend on the antigen and the site of immunization. Drainage is reduced in response to inflammation caused by oxazolone skin painting and lipopolysaccharide in peritoneal models. However, drainage increases when complete Freund's adjuvant is applied to the mouse foot pad.38,72,73 The contradiction between the expansion of the lymphatic network and the reduction of lymphatic drainage in some models may be explained by the regulation of collecting lymphatic vessel function during inflammation. Accumulated macrophages can also promote lymphangiogenesis by expressing multiple lymphangiogenic growth factors such as VEGF and VEGF-C.74–76 B cells also enhance lymphangiogenesis, but T cells appear to inhibit lymphangiogenesis during inflammation.38,72,77,78
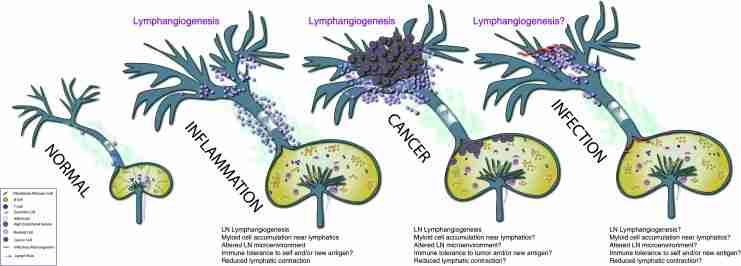
Alterations to the lymphatic system in pathological conditions. (A) The normal lymphatic system consists of lymphatic capillaries that form lymph, collecting lymphatic vessels that transport lymph, and lymph nodes that organize immune responses. Each of these structures has its own unique microarchitecture that contributes to the maintenance of proper immune function. (B) During inflammation, lymphangiogenesis is seen in the lymphatic capillaries as well as in the local draining lymph node. Furthermore, a robust infiltrate of myeloid-derived cells accumulate around the collecting lymphatic vessels, which is correlated with suppression of lymphatic contractions and weakened immune response. (C) During tumor growth, lymphangiogenesis is also seen in the tumor periphery and surrounding tissue. Furthermore, robust lymphangiogenesis has been observed in the lymph node, along with architectural changes associated with creating an environment receptive to tumor metastases. The ability of tumors to alter collecting lymphatic vessel contraction is not known. (D) Little is known about the response of lymphatic vessels to microorganisms or viral infections. However, there is a strong clinical link between infectious disease and lymphedema. This area needs much greater scrutiny to identify new opportunities to treat infections that are difficult to clear.
Temporally and spatially dependent production of NO by activated eNOS in LECs is required for the contraction of collecting lymphatic vessels under physiological conditions, while NO produced by iNOS during inflammation attenuates lymphatic contraction strength by overwhelming these critical NO gradients. Induction of cutaneous inflammation in mice by oxazalone skin painting results in infiltration of iNOS-expressing, CD11b+Gr-1+ myeloid cells from the bone marrow, resulting in locally elevated levels of NO. iNOS depletion in these cells allows the temporal and spatial NO gradients to be maintained by eNOS and prevents the attenuation of lymphatic contractions. These experiments underscore a mechanism by which immune cell infiltrates alter lymph flow.22,23 iNOS-mediated suppression of lymphatic contraction and dilation of lymphatic vessels is independent of cell type. Ex vivo activation of macrophages suppresses lymphatic contraction in vivo and ex vivo at a level similar to that seen in iNOS-expressing bone marrow derived cells stimulated by oxazalone.23,79 More importantly, the interruption of lymphatic contraction during acute inflammation is associated with the prevention of self-antigen induced autoimmunity.23 Collecting lymphatic vessels and lymph nodes are embedded in adipose tissue. Accumulation of fat tissue and macrophage infiltration are associated with the progression of inflammation, and both may contribute to the suppression of collecting lymphatic vessel contractility.
Lymph nodes appear to be more tolerogenic to new antigens during inflammation. The draining LN structure undergoes dramatic changes during local inflammation, including angiogenesis, lymphangiogenesis, decreased expression of genes contributing to PNAd, and decreased chemokine production of CCL21 and CXCL13.38,39,42,77,80–82 These temporary changes in the LN hinder naïve T-cell and DC access to the node, and thus limit their interactions. The ability of effector cells to leave the LN is also enhanced, thus preventing pathological immune-mediated damage in the LN. One can speculate that these changes in the lymph node are intended to maintain self-tolerance during a non-infectious inflammatory process. However, these changes in the lymph node may also prevent an immune response to subsequent bacterial or viral infection. It has been shown that disruption of lymphatic vessels impairs fluid drainage and the body's ability to activate an immune response to a pathogen, which leads to persistent infection.38,83–87 During clinical lymphedema, immune cell accumulation and impaired immune response are also frequently observed.88
Chronic inflammation can lead to the formation of tertiary lymphoid organs (TLOs), which recapitulate key features of LNs. The formation of TLOs is associated with lymphangiogenesis induced by chronic allograft rejection, autoimmune reactions, or microbial infections. A substantial increase in lymphatic vessel density associated with immune cell infiltrates can be found in human kidney transplants that are rejected.89 Additionally, corneal graft rejection can be predicted by the presence of lymphangiogenesis.90 In rheumatoid arthritis patients, the lymphangiogenic factor VEGF-C is increased in the synovium.91 Lymphangiogenesis is also associated with autoimmune conditions in the gastrointestinal tract. In addition to lymphangiogenesis, collecting lymphatic contraction is found to be reduced in models of inflammatory bowel disease.92 Factors such as macrophages, mast cells, iNOS, and prostaglandins contribute to this suppression of lymphatic contraction.92 Other inflammatory cytokines and growth factors produced during inflammation may also affect collecting lymphatic function. The role of cytokines, chemokines, and growth factors in lymphatic contraction remains an area of research focus.
Lymphatics in Cancer Progression
Cancer progression shares many features of wound healing and inflammatory conditions, including angiogenesis,38,72,77 lymphangiogenesis,93,94 and immune cell recruitment95,96 (Fig. 1). Tumor-induced lymphangiogenesis provides an expanded lymphatic network that enhances molecular and cell delivery to the draining LN.97 In fact, lymphangiogenesis correlates with increased lymph node metastasis and poor prognosis of cancer patients.88,98–101 However, the role of lymphangiogenesis in immune function during cancer progression is not well understood.
Upon cytotoxic treatment—such as radiation and chemotherapy—large numbers of tumor cells die through apoptotic pathways. Rather than triggering an anti-tumor immune response, these apoptotic cells in general induce immune tolerance to their associated antigens.102,103 However, vaccination with an injection of lethally-irradiated tumor cells induces CD8+ cytotoxic T cell activation and prevents subsequent syngeneic tumor growth.104 The injected apoptotic cells travel directly to the lymph node and are picked up by macrophages in the lymph node sinus. The macrophages then cross-present tumor antigen to DCs or directly present antigen to cytotoxic T cells, activating a response.104 These contradictory results from cytotoxic treatment and vaccination suggest that the route or kinetics of clearance of material from apoptotic cells may determine the ultimate immune response.
Lymphangiogenesis occurs within the tumor-draining LN prior to tumor seeding94 and is thought to be preparing the “soil” for metastatic cells (“the seed”). The growth of metastatic cancer cells in LNs is a critical event in disease progression that profoundly impacts patient prognosis and treatment decisions.105,106 The poor prognosis for patients with lymph node metastasis may be due to immune tolerance caused by the primary tumor. However, these effects are not well characterized. It is possible that chronic inflammatory mediators induced by the tumors, tumor secreted factors or tumor antigen from apoptotic tumor cells can induce peripheral tolerance. In addition, lymphatic endothelial cells can present tumor antigen and may induce immune tolerance.107 All of these factors need to be considered in the development of immune therapy for metastatic tumors.
Interstitial fluid pressure in cancer is frequently increased as a result of the hyperpermeable tumor blood vasculature and the lack of lymphatic function from inside tumors.108 Interstitial hypertension in tumors increases interstitial flow leaving the tumor, which has been positively correlated with cancer cell dissemination to the draining lymph node.107,109 Cancer cells invading the tumor margin enter enlarged lymphatic vessels and travel with the lymph flow through the collecting lymphatic vessels to the draining LN.110 However, it is likely that the function of collecting lymphatic vessels draining the tumor is impaired, similar to its inhibition during inflammatory conditions. How this impairment inhibits the arrival of antigen in the lymph node and the subsequent immune response is unknown.
Lymphatics in Infectious Diseases
Disruption of lymphatic function leads to lymphedema, the consequences of which are disfigurement and compromised immune defenses in the affected region. Worldwide, infectious diseases are the major causes of acquired lymphedema. However, it is not clear how infectious microorganisms affect lymphatic function. Upon infection, antigen from the bacteria or viral proteins can be picked up by DCs to be transported to the lymph node. Viruses and bacteria are also small enough to pass through the endothelium of lymphatic capillaries and travel through collecting lymphatic vessels to enter the node. Here, they are sampled by sinus macrophages and B cells.52–54 Additionally, sinus macrophages prevent pathogens from exiting to the efferent lymph without being interrogated—depleting sinus macrophages leads to systemic infections.55 Worth noting, most experimental models of infectious disease use needle injections, which mimic vaccination. However, many infectious microorganisms, such as influenza virus or Herpes simplex virus-1 (HSV), infect mucosal tissues and need to disrupt the epithelial layer before entering interstitial spaces. Thus, the infection route may determine the efficiency of immune responses initiated in the draining lymph node. Needle-injected HSV-1 will become a lymph-borne antigen and is subsequently presented by resident DCs in the lymph node. In contrast, HSV that infects the vaginal mucosa is presented by tissue migrating DCs.111 Both migrating and LN resident DCs are required to process the antigen and activate efficient CD4 T cell priming, but their functions are different.57 Lymph node resident DCs activate T cells and retain the antigen-specific T cell in the lymph node. Tissue-migrating DCs, which arrive after the initial infection, induce antigen-specific T cell proliferation.57 How lymphatic function is affected by infection, and conversely, how alterations in lymphatic function affect immune responses are not clear. However, disruption of lymphatic vessels inhibits the drainage function, leads to lymphedema and reduces antigen transport to the lymph node.87 The stagnant fluid in lymphedematous tissue can become a further nidus for infection, thus exacerbating the already immuno-compromised state of the affected tissue. In addition, larger microorganisms, such as mosquito-transmitted filarial parasites, may be trapped and proliferate in lymphatic vessels, blocking lymph flow.112 These examples suggest that lymphatic transport and function of the lymphatic system are tied to immune response, but to date this area of research has been underexplored.
Conclusions
The lymphatic system maintains host peripheral tolerance during normal conditions, and quickly initiates protective immunity against foreign antigens upon stimulation. The lymphatic capillaries, collecting lymphatic vessels, and lymph nodes coordinate efficient antigen delivery, antigen presentation, and cell–cell interaction. Many pathological conditions cause lymphangiogenesis, presumably providing an expanded lymphatic network that allows greater access for antigen and fluid to enter lymphatic vessels. Lymphatic function is also altered in various disease states. However, the role of lymphangiogenesis and changes in lymphatic function in immune regulation is not clear. For example, malignant tumors can cause local lymphangiogenesis via increasing production of VEGFC/D, allowing cancer cells to invade and be transported via the lymphatic drainage to the lymph node. Metastatic tumors can also induce lymph node lymphangiogenesis. On one hand, tumor lymphangiogenesis increases lymph flow, suggesting it may increase tumor antigen delivery to the tumor draining lymph node. On the other hand, tumor lymphangiogenesis carries with it a poor prognosis,105,106 which may be partially due to tumor-induced immune tolerance as well as an increased ability of the cancer cells to travel to the LN. Similarly contradictory predictions of lymphangiogenesis between enhancing immunity and immune tolerance are also seen in transplant rejection. Blocking lymphangiogenesis reduces the rate of acute transplant rejection, but one-year follow-up data indicate that continued function of the transplanted organ is correlated with lymphangiogenesis.113–115 In addition, a viral infection generates anti-viral immunity but also causes suppression of the bystander CD8 T cell responses through inducing tolerogenic gene expression in lymph node stromal cells.82,116 How the lymphatic system is regulated in a diverse set of diseases and how its function exerts its influence on ultimate immune function needs further study. Understanding the connection between lymphatic function and immune regulation will lead to new therapeutic opportunities in cancer, infection, and autoimmune diseases.
Acknowledgments
We thank Drs. Mark Badeaux, Cristina Kesler and Lance Munn for their critical input on this manuscript.
Author Disclosure Statement
The authors have no financial conflicts of interest.
The authors are supported by NIH R00CA137167, NIH DP2OD008780, NIH R21AI097745 and NCI Federal Share/Proton Beam Income (TPP), Charles King Trust Fellowship and NIH K99HL111343 (SL).
References
Articles from Lymphatic Research and Biology are provided here courtesy of Mary Ann Liebert, Inc.