Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain
- PMID: 24227727
- PMCID: PMC3866416
- DOI: 10.1523/JNEUROSCI.1592-13.2013
Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain
Abstract
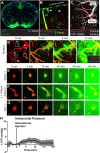
CSF from the subarachnoid space moves rapidly into the brain along paravascular routes surrounding penetrating cerebral arteries, exchanging with brain interstitial fluid (ISF) and facilitating the clearance of interstitial solutes, such as amyloid β, in a pathway that we have termed the "glymphatic" system. Prior reports have suggested that paravascular bulk flow of CSF or ISF may be driven by arterial pulsation. However, cerebral arterial pulsation could not be directly assessed. In the present study, we use in vivo two-photon microscopy in mice to visualize vascular wall pulsatility in penetrating intracortical arteries. We observed that unilateral ligation of the internal carotid artery significantly reduced arterial pulsatility by ~50%, while systemic administration of the adrenergic agonist dobutamine increased pulsatility of penetrating arteries by ~60%. When paravascular CSF-ISF exchange was evaluated in real time using in vivo two-photon and ex vivo fluorescence imaging, we observed that internal carotid artery ligation slowed the rate of paravascular CSF-ISF exchange, while dobutamine increased the rate of paravascular CSF-ISF exchange. These findings demonstrate that cerebral arterial pulsatility is a key driver of paravascular CSF influx into and through the brain parenchyma, and suggest that changes in arterial pulsatility may contribute to accumulation and deposition of toxic solutes, including amyloid β, in the aging brain.
Figures
Similar articles
-
Impairment of paravascular clearance pathways in the aging brain.Ann Neurol. 2014 Dec;76(6):845-61. doi: 10.1002/ana.24271. Epub 2014 Sep 26. Ann Neurol. 2014. PMID: 25204284 Free PMC article.
-
A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β.Sci Transl Med. 2012 Aug 15;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. Sci Transl Med. 2012. PMID: 22896675 Free PMC article.
-
Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache.J Neurosci. 2017 Mar 15;37(11):2904-2915. doi: 10.1523/JNEUROSCI.3390-16.2017. Epub 2017 Feb 13. J Neurosci. 2017. PMID: 28193695 Free PMC article.
-
Paravascular spaces: entry to or exit from the brain?Exp Physiol. 2019 Jul;104(7):1013-1017. doi: 10.1113/EP087424. Epub 2019 Jan 9. Exp Physiol. 2019. PMID: 30582766 Free PMC article. Review.
-
The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy.Front Neuroanat. 2017 Nov 7;11:101. doi: 10.3389/fnana.2017.00101. eCollection 2017. Front Neuroanat. 2017. PMID: 29163074 Free PMC article. Review.
Cited by 218 articles
-
Echocardiographic index E/e' in association with cerebral white matter hyperintensity progression.PLoS One. 2020 Jul 27;15(7):e0236473. doi: 10.1371/journal.pone.0236473. eCollection 2020. PLoS One. 2020. PMID: 32716979 Free PMC article.
-
Arterial pulsations drive oscillatory flow of CSF but not directional pumping.Sci Rep. 2020 Jun 22;10(1):10102. doi: 10.1038/s41598-020-66887-w. Sci Rep. 2020. PMID: 32572120 Free PMC article.
-
Mechanisms behind altered pulsatile intracranial pressure in idiopathic normal pressure hydrocephalus: role of vascular pulsatility and systemic hemodynamic variables.Acta Neurochir (Wien). 2020 Aug;162(8):1803-1813. doi: 10.1007/s00701-020-04423-5. Epub 2020 Jun 12. Acta Neurochir (Wien). 2020. PMID: 32533412 Free PMC article.
-
Magnetic Resonance Imaging and Modeling of the Glymphatic System.Diagnostics (Basel). 2020 May 27;10(6):344. doi: 10.3390/diagnostics10060344. Diagnostics (Basel). 2020. PMID: 32471025 Free PMC article. Review.
-
Most Small Cerebral Cortical Veins Demonstrate Significant Flow Pulsatility: A Human Phase Contrast MRI Study at 7T.Front Neurosci. 2020 May 5;14:415. doi: 10.3389/fnins.2020.00415. eCollection 2020. Front Neurosci. 2020. PMID: 32431591 Free PMC article.
Publication types
MeSH terms
Grant support
LinkOut - more resources
-
Full Text Sources
-
Other Literature Sources
