Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer
- PMID: 23635358
- PMCID: PMC3665671
- DOI: 10.1186/1479-5876-11-107
Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer
Abstract
Background: Neurodegenerative diseases such as Alzheimer's are associated with the aggregation of endogenous peptides and proteins that contribute to neuronal dysfunction and loss. The glymphatic system, a brain-wide perivascular pathway along which cerebrospinal fluid (CSF) and interstitial fluid (ISF) rapidly exchange, has recently been identified as a key contributor to the clearance of interstitial solutes from the brain, including amyloid β. These findings suggest that measuring changes in glymphatic pathway function may be an important prognostic for evaluating neurodegenerative disease susceptibility or progression. However, no clinically acceptable approach to evaluate glymphatic pathway function in humans has yet been developed.
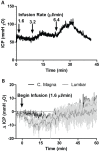
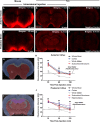
Methods: Time-sequenced ex vivo fluorescence imaging of coronal rat and mouse brain slices was performed at 30-180 min following intrathecal infusion of CSF tracer (Texas Red- dextran-3, MW 3 kD; FITC- dextran-500, MW 500 kD) into the cisterna magna or lumbar spine. Tracer influx into different brain regions (cortex, white matter, subcortical structures, and hippocampus) in rat was quantified to map the movement of CSF tracer following infusion along both routes, and to determine whether glymphatic pathway function could be evaluated after lumbar intrathecal infusion.
Results: Following lumbar intrathecal infusions, small molecular weight TR-d3 entered the brain along perivascular pathways and exchanged broadly with the brain ISF, consistent with the initial characterization of the glymphatic pathway in mice. Large molecular weight FITC-d500 remained confined to the perivascular spaces. Lumbar intrathecal infusions exhibited a reduced and delayed peak parenchymal fluorescence intensity compared to intracisternal infusions.
Conclusion: Lumbar intrathecal contrast delivery is a clinically useful approach that could be used in conjunction with dynamic contrast enhanced MRI nuclear imaging to assess glymphatic pathway function in humans.
Figures
Similar articles
-
Brain-wide pathway for waste clearance captured by contrast-enhanced MRI.J Clin Invest. 2013 Mar;123(3):1299-309. doi: 10.1172/JCI67677. Epub 2013 Feb 22. J Clin Invest. 2013. PMID: 23434588 Free PMC article.
-
Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport.Neuroimage. 2017 May 15;152:530-537. doi: 10.1016/j.neuroimage.2017.03.021. Epub 2017 Mar 18. Neuroimage. 2017. PMID: 28323163 Free PMC article.
-
Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts.J Neurosci. 2017 Mar 15;37(11):2870-2877. doi: 10.1523/JNEUROSCI.2112-16.2017. Epub 2017 Feb 10. J Neurosci. 2017. PMID: 28188218 Free PMC article.
-
A new glaucoma hypothesis: a role of glymphatic system dysfunction.Fluids Barriers CNS. 2015 Jun 29;12:16. doi: 10.1186/s12987-015-0012-z. Fluids Barriers CNS. 2015. PMID: 26118970 Free PMC article. Review.
-
The role of brain barriers in fluid movement in the CNS: is there a 'glymphatic' system?Acta Neuropathol. 2018 Mar;135(3):387-407. doi: 10.1007/s00401-018-1812-4. Epub 2018 Feb 10. Acta Neuropathol. 2018. PMID: 29428972 Review.
Cited by 95 articles
-
The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease.Cell. 2020 Jul 23;182(2):270-296. doi: 10.1016/j.cell.2020.06.039. Cell. 2020. PMID: 32707093 Review.
-
Arterial pulsations drive oscillatory flow of CSF but not directional pumping.Sci Rep. 2020 Jun 22;10(1):10102. doi: 10.1038/s41598-020-66887-w. Sci Rep. 2020. PMID: 32572120 Free PMC article.
-
Microglial and Astrocytic Function in Physiological and Pathological Conditions: Estrogenic Modulation.Int J Mol Sci. 2020 May 2;21(9):3219. doi: 10.3390/ijms21093219. Int J Mol Sci. 2020. PMID: 32370112 Free PMC article. Review.
-
Intracranial pressure elevation alters CSF clearance pathways.Fluids Barriers CNS. 2020 Apr 16;17(1):29. doi: 10.1186/s12987-020-00189-1. Fluids Barriers CNS. 2020. PMID: 32299464 Free PMC article.
-
Repetitive Mild Traumatic Brain Injury Alters Glymphatic Clearance Rates in Limbic Structures of Adolescent Female Rats.Sci Rep. 2020 Apr 10;10(1):6254. doi: 10.1038/s41598-020-63022-7. Sci Rep. 2020. PMID: 32277097 Free PMC article.
References
-
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–1854. - PubMed
