Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo
- PMID: 22536283
- PMCID: PMC3318905
- DOI: 10.1155/2012/429320
Anti-Inflammatory Activities of Cinnamomum cassia Constituents In Vitro and In Vivo
Abstract
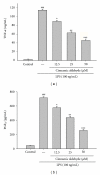
We have investigated the anti-inflammatory effects of Cinnamomum cassia constituents (cinnamic aldehyde, cinnamic alcohol, cinnamic acid, and coumarin) using lipopolysaccharide (LPS)-stimulated mouse macrophage (RAW264.7) and carrageenan (Carr)-induced mouse paw edema model. When RAW264.7 macrophages were treated with cinnamic aldehyde together with LPS, a significant concentration-dependent inhibition of nitric oxide (NO), tumor necrosis factor (TNF-α), and prostaglandin E2 (PGE(2)) levels productions were detected. Western blotting revealed that cinnamic aldehyde blocked protein expression of inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), nuclear transcription factor kappa B (NF-κB), and IκBα, significantly. In the anti-inflammatory test, cinnamic aldehyde decreased the paw edema after Carr administration, and increased the activities of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in the paw tissue. We also demonstrated cinnamic aldehyde attenuated the malondialdehyde (MDA) level and myeloperoxidase (MPO) activity in the edema paw after Carr injection. Cinnamic aldehyde decreased the NO, TNF-α, and PGE(2) levels on the serum level after Carr injection. Western blotting revealed that cinnamic aldehyde decreased Carr-induced iNOS, COX-2, and NF-κB expressions in the edema paw. These findings demonstrated that cinnamic aldehyde has excellent anti-inflammatory activities and thus has great potential to be used as a source for natural health products.
Figures
Similar articles
-
Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo.PLoS One. 2012;7(5):e35922. doi: 10.1371/journal.pone.0035922. Epub 2012 May 8. PLoS One. 2012. PMID: 22590514 Free PMC article.
-
Involvement of Heme Oxygenase-1 Participates in Anti-Inflammatory and Analgesic Effects of Aqueous Extract of Hibiscus taiwanensis.Evid Based Complement Alternat Med. 2012;2012:132859. doi: 10.1155/2012/132859. Epub 2012 Jun 21. Evid Based Complement Alternat Med. 2012. PMID: 22778769 Free PMC article.
-
Anti-inflammatory activities of 6β-acetoxy-7α-hydroxyroyleanone from Taiwania cryptomerioides Hayata ex vivo and in vivo.J Agric Food Chem. 2011 Oct 26;59(20):11211-8. doi: 10.1021/jf200576f. Epub 2011 Sep 27. J Agric Food Chem. 2011. PMID: 21830830
-
Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory activity of imperatorin from Glehnia littoralis.J Agric Food Chem. 2012 Feb 22;60(7):1673-81. doi: 10.1021/jf204297e. Epub 2012 Feb 8. J Agric Food Chem. 2012. PMID: 22188242
-
Anti-inflammatory effects of trilinolein from Panax notoginseng through the suppression of NF-κB and MAPK expression and proinflammatory cytokine expression.Am J Chin Med. 2014;42(6):1485-506. doi: 10.1142/S0192415X14500931. Am J Chin Med. 2014. PMID: 25482678
Cited by 55 articles
-
Clinical Efficacy and Safety of Yellow Oil Formulations 3 and 4 versus Indomethacin Solution in Patients with Symptomatic Osteoarthritis of the Knee: A Randomized Controlled Trial.Evid Based Complement Alternat Med. 2020 Jul 25;2020:5782178. doi: 10.1155/2020/5782178. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32774422 Free PMC article.
-
Evaluation of changes in cytochrome P450 2C19 activity in type 2 diabetic rats before and after treatment, by using isolated perfused liver model.Iran J Basic Med Sci. 2020 May;23(5):629-635. doi: 10.22038/ijbms.2020.40836.9642. Iran J Basic Med Sci. 2020. PMID: 32742601 Free PMC article.
-
Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study.J Diabetes Metab Disord. 2019 Dec 23;19(1):53-60. doi: 10.1007/s40200-019-00474-3. eCollection 2020 Jun. J Diabetes Metab Disord. 2019. PMID: 32550156
-
Cinnamaldehyde, a Promising Natural Preservative Against Aspergillus flavus.Front Microbiol. 2019 Dec 18;10:2895. doi: 10.3389/fmicb.2019.02895. eCollection 2019. Front Microbiol. 2019. PMID: 31921070 Free PMC article.
-
Efficacy, safety, and cost-effectiveness analysis of adjuvant herbal medicine treatment, Palmijihwang-hwan, for chronic low back pain: a study protocol for randomized, controlled, assessor-blinded, multicenter clinical trial.Trials. 2019 Dec 27;20(1):778. doi: 10.1186/s13063-019-3776-7. Trials. 2019. PMID: 31882016 Free PMC article.
References
-
- Fang SC, Hsu CL, Yen GC. Anti-inflammatory effects of phenolic compounds isolated from the fruits of Artocarpus heterophyllus . Journal of Agricultural and Food Chemistry. 2008;56(12):4463–4468. - PubMed
-
- Huang M-H, Wang B-S, Chiu C-S, et al. Antioxidant, antinociceptive, and anti-inflammatory activities of Xanthii Fructus extract. Journal of Ethnopharmacology. 2011;135(2):545–552. - PubMed
-
- Chang C-T, Huang S-S, Lin S-S, et al. Anti-inflammatory activities of tormentic acid from suspension cells of Eriobotrya Japonica ex vivo and in vivo . Food Chemistry. 2011;127(3):1131–1137. - PubMed
LinkOut - more resources
-
Full Text Sources
-
Other Literature Sources
-
Medical
-
Miscellaneous
