Propagation of tau misfolding from the outside to the inside of a cell
- PMID: 19282288
- PMCID: PMC2676015
- DOI: 10.1074/jbc.M808759200
Propagation of tau misfolding from the outside to the inside of a cell
Abstract
Tauopathies are neurodegenerative diseases characterized by aggregation of the microtubule-associated protein Tau in neurons and glia. Although Tau is normally considered an intracellular protein, Tau aggregates are observed in the extracellular space, and Tau peptide is readily detected in the cerebrospinal fluid of patients. Tau aggregation occurs in many diseases, including Alzheimer disease and frontotemporal dementia. Tau pathology begins in discrete, disease-specific regions but eventually involves much larger areas of the brain. It is unknown how this propagation of Tau misfolding occurs. We hypothesize that extracellular Tau aggregates can transmit a misfolded state from the outside to the inside of a cell, similar to prions. Here we show that extracellular Tau aggregates, but not monomer, are taken up by cultured cells. Internalized Tau aggregates displace tubulin, co-localize with dextran, a marker of fluid-phase endocytosis, and induce fibrillization of intracellular full-length Tau. These intracellular fibrils are competent to seed fibril formation of recombinant Tau monomer in vitro. Finally, we observed that newly aggregated intracellular Tau transfers between co-cultured cells. Our data indicate that Tau aggregates can propagate a fibrillar, misfolded state from the outside to the inside of a cell. This may have important implications for understanding how protein misfolding spreads through the brains of tauopathy patients, and it is potentially relevant to myriad neurodegenerative diseases associated with protein misfolding.
Figures
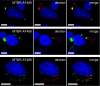
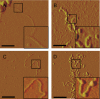
Similar articles
-
Trans-cellular propagation of Tau aggregation by fibrillar species.J Biol Chem. 2012 Jun 1;287(23):19440-51. doi: 10.1074/jbc.M112.346072. Epub 2012 Mar 29. J Biol Chem. 2012. PMID: 22461630 Free PMC article.
-
Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons.J Biol Chem. 2013 Jan 18;288(3):1856-70. doi: 10.1074/jbc.M112.394528. Epub 2012 Nov 27. J Biol Chem. 2013. PMID: 23188818 Free PMC article.
-
Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds.Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):E3138-47. doi: 10.1073/pnas.1301440110. Epub 2013 Jul 29. Proc Natl Acad Sci U S A. 2013. PMID: 23898162 Free PMC article.
-
Mechanisms of secretion and spreading of pathological tau protein.Cell Mol Life Sci. 2020 May;77(9):1721-1744. doi: 10.1007/s00018-019-03349-1. Epub 2019 Oct 30. Cell Mol Life Sci. 2020. PMID: 31667556 Free PMC article. Review.
-
[Can prion-like propagation occur in neurodegenerative diseases?: in view of transmissible systemic amyloidosis].Brain Nerve. 2012 Jun;64(6):665-74. Brain Nerve. 2012. PMID: 22647474 Review. Japanese.
Cited by 445 articles
-
Human Serum Albumin-Inspired Glycopeptide-Based Multifunctional Inhibitor of Amyloid-β Toxicity.ACS Omega. 2020 Jul 20;5(30):18628-18641. doi: 10.1021/acsomega.0c01028. eCollection 2020 Aug 4. ACS Omega. 2020. PMID: 32775865 Free PMC article.
-
Intramuscular injection of vectorized-scFvMC1 reduces pathological tau in two different tau transgenic models.Acta Neuropathol Commun. 2020 Aug 6;8(1):126. doi: 10.1186/s40478-020-01003-7. Acta Neuropathol Commun. 2020. PMID: 32762731 Free PMC article.
-
Revealing the Proteome of Motor Cortex Derived Extracellular Vesicles Isolated from Amyotrophic Lateral Sclerosis Human Postmortem Tissues.Cells. 2020 Jul 16;9(7):1709. doi: 10.3390/cells9071709. Cells. 2020. PMID: 32708779 Free PMC article.
-
Red Ginseng Inhibits Tau Aggregation and Promotes Tau Dissociation In Vitro.Oxid Med Cell Longev. 2020 Jun 30;2020:7829842. doi: 10.1155/2020/7829842. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32685100 Free PMC article.
-
Paired Helical Filament-Forming Region of Tau (297-391) Influences Endogenous Tau Protein and Accumulates in Acidic Compartments in Human Neuronal Cells.J Mol Biol. 2020 Aug 7;432(17):4891-4907. doi: 10.1016/j.jmb.2020.05.027. Epub 2020 Jul 16. J Mol Biol. 2020. PMID: 32681841 Free PMC article.
Publication types
MeSH terms
Substances
Grant support
LinkOut - more resources
-
Full Text Sources
-
Other Literature Sources
-
Medical
