Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production
- PMID: 18923182
- PMCID: PMC2909187
- DOI: 10.1152/physrev.00031.2007
Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production
Abstract
The first suggestion that physical exercise results in free radical-mediated damage to tissues appeared in 1978, and the past three decades have resulted in a large growth of knowledge regarding exercise and oxidative stress. Although the sources of oxidant production during exercise continue to be debated, it is now well established that both resting and contracting skeletal muscles produce reactive oxygen species and reactive nitrogen species. Importantly, intense and prolonged exercise can result in oxidative damage to both proteins and lipids in the contracting myocytes. Furthermore, oxidants can modulate a number of cell signaling pathways and regulate the expression of multiple genes in eukaryotic cells. This oxidant-mediated change in gene expression involves changes at transcriptional, mRNA stability, and signal transduction levels. Furthermore, numerous products associated with oxidant-modulated genes have been identified and include antioxidant enzymes, stress proteins, DNA repair proteins, and mitochondrial electron transport proteins. Interestingly, low and physiological levels of reactive oxygen species are required for normal force production in skeletal muscle, but high levels of reactive oxygen species promote contractile dysfunction resulting in muscle weakness and fatigue. Ongoing research continues to probe the mechanisms by which oxidants influence skeletal muscle contractile properties and to explore interventions capable of protecting muscle from oxidant-mediated dysfunction.
Figures
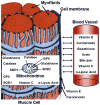
Comment in
-
Parallels of snipe hunting and ROS research: the challenges of studying ROS and redox signalling in response to exercise.J Physiol. 2009 Mar 1;587(Pt 5):927-8. doi: 10.1113/jphysiol.2008.165605. Epub 2009 Jan 5. J Physiol. 2009. PMID: 19124537 Free PMC article. Review. No abstract available.
Similar articles
-
Reactive oxygen species: impact on skeletal muscle.Compr Physiol. 2011 Apr;1(2):941-69. doi: 10.1002/cphy.c100054. Compr Physiol. 2011. PMID: 23737208 Free PMC article. Review.
-
Interplay of oxidants and antioxidants during exercise: implications for muscle health.Phys Sportsmed. 2009 Dec;37(4):116-23. doi: 10.3810/psm.2009.12.1749. Phys Sportsmed. 2009. PMID: 20048548 Review.
-
Exercise-induced oxidative stress in humans: cause and consequences.Free Radic Biol Med. 2011 Sep 1;51(5):942-50. doi: 10.1016/j.freeradbiomed.2010.12.009. Epub 2010 Dec 16. Free Radic Biol Med. 2011. PMID: 21167935 Review.
-
Mediators of Physical Activity Protection against ROS-Linked Skeletal Muscle Damage.Int J Mol Sci. 2019 Jun 20;20(12):3024. doi: 10.3390/ijms20123024. Int J Mol Sci. 2019. PMID: 31226872 Free PMC article. Review.
-
Effects of reactive oxygen species and interplay of antioxidants during physical exercise in skeletal muscles.J Physiol Biochem. 2018 Aug;74(3):359-367. doi: 10.1007/s13105-018-0633-1. Epub 2018 May 1. J Physiol Biochem. 2018. PMID: 29713940 Review.
Cited by 558 articles
-
Oral treatment with the Chinese herbal supplements B307 enhances muscle endurance of ICR mice after exhaustive swimming via suppressing fatigue, oxidative stress, and inflammation.Food Sci Nutr. 2020 May 19;8(7):3682-3691. doi: 10.1002/fsn3.1652. eCollection 2020 Jul. Food Sci Nutr. 2020. PMID: 32724631 Free PMC article.
-
Assessment of Dietary Intake and Nutritional Status in CrossFit-Trained Individuals: A Descriptive Study.Int J Environ Res Public Health. 2020 Jul 2;17(13):4772. doi: 10.3390/ijerph17134772. Int J Environ Res Public Health. 2020. PMID: 32630749 Free PMC article.
-
Outcomes Assessment of Sustainable and Innovatively Simple Lifestyle Modification at the Workplace - Drinking Electrolyzed-Reduced Water (OASIS-ERW): A Randomized, Double-Blind, Placebo-Controlled Trial.Antioxidants (Basel). 2020 Jun 27;9(7):564. doi: 10.3390/antiox9070564. Antioxidants (Basel). 2020. PMID: 32605142 Free PMC article.
-
Total Dietary Antioxidant Intake Including Polyphenol Content: Is it Capable to Fight against Increased Oxidants within the Body of Ultra-Endurance Athletes?Nutrients. 2020 Jun 23;12(6):1877. doi: 10.3390/nu12061877. Nutrients. 2020. PMID: 32586010 Free PMC article.
-
Organosulfur Compounds: A Review of Their Anti-inflammatory Effects in Human Health.Front Nutr. 2020 Jun 2;7:64. doi: 10.3389/fnut.2020.00064. eCollection 2020. Front Nutr. 2020. PMID: 32582751 Free PMC article. Review.
Publication types
MeSH terms
Substances
Grant support
LinkOut - more resources
-
Full Text Sources
-
Other Literature Sources
-
Medical
-
Miscellaneous
