The Glymphatic System: A Beginner's Guide
- PMID: 25947369
- PMCID: PMC4636982
- DOI: 10.1007/s11064-015-1581-6
The Glymphatic System: A Beginner's Guide
Abstract
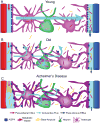
The glymphatic system is a recently discovered macroscopic waste clearance system that utilizes a unique system of perivascular tunnels, formed by astroglial cells, to promote efficient elimination of soluble proteins and metabolites from the central nervous system. Besides waste elimination, the glymphatic system also facilitates brain-wide distribution of several compounds, including glucose, lipids, amino acids, growth factors, and neuromodulators. Intriguingly, the glymphatic system function mainly during sleep and is largely disengaged during wakefulness. The biological need for sleep across all species may therefore reflect that the brain must enter a state of activity that enables elimination of potentially neurotoxic waste products, including β-amyloid. Since the concept of the glymphatic system is relatively new, we will here review its basic structural elements, organization, regulation, and functions. We will also discuss recent studies indicating that glymphatic function is suppressed in various diseases and that failure of glymphatic function in turn might contribute to pathology in neurodegenerative disorders, traumatic brain injury and stroke.
Keywords: Aging; Astrocytes; Cerebrospinal fluid secretion; Neurodegenerative diseases; Perivascular spaces; Sleep; The glymphatic system; Traumatic brain injury; Virchow–Robin spaces.
Conflict of interest statement
Figures


Similar articles
-
Glymphatic clearance controls state-dependent changes in brain lactate concentration.J Cereb Blood Flow Metab. 2017 Jun;37(6):2112-2124. doi: 10.1177/0271678X16661202. Epub 2016 Jan 1. J Cereb Blood Flow Metab. 2017. PMID: 27481936 Free PMC article.
-
[The glymphatic system: concept, function and research progresses].Sheng Li Xue Bao. 2018 Feb 25;70(1):52-60. Sheng Li Xue Bao. 2018. PMID: 29492515 Review. Chinese.
-
The Glymphatic-Lymphatic Continuum: Opportunities for Osteopathic Manipulative Medicine.J Am Osteopath Assoc. 2016 Mar;116(3):170-7. doi: 10.7556/jaoa.2016.033. J Am Osteopath Assoc. 2016. PMID: 26927910 Review.
-
Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases.Rejuvenation Res. 2013 Dec;16(6):518-23. doi: 10.1089/rej.2013.1530. Rejuvenation Res. 2013. PMID: 24199995 Review.
-
Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy.Neurosci Biobehav Rev. 2018 Jan;84:316-324. doi: 10.1016/j.neubiorev.2017.08.016. Epub 2017 Aug 30. Neurosci Biobehav Rev. 2018. PMID: 28859995 Review.
Cited by 213 articles
-
Omega-3 Polyunsaturated Fatty Acids Alleviate Traumatic Brain Injury by Regulating the Glymphatic Pathway in Mice.Front Neurol. 2020 Jul 17;11:707. doi: 10.3389/fneur.2020.00707. eCollection 2020. Front Neurol. 2020. PMID: 32765412 Free PMC article.
-
A Review of Hematoma Components Clearance Mechanism After Subarachnoid Hemorrhage.Front Neurosci. 2020 Jul 7;14:685. doi: 10.3389/fnins.2020.00685. eCollection 2020. Front Neurosci. 2020. PMID: 32733194 Free PMC article. Review.
-
Location matters: highly divergent protein levels in samples from different CNS compartments in a clinical trial of rituximab for progressive MS.Fluids Barriers CNS. 2020 Jul 29;17(1):49. doi: 10.1186/s12987-020-00205-4. Fluids Barriers CNS. 2020. PMID: 32727487 Free PMC article.
-
Potential pharmacological approaches for the treatment of HIV-1 associated neurocognitive disorders.Fluids Barriers CNS. 2020 Jul 10;17(1):42. doi: 10.1186/s12987-020-00204-5. Fluids Barriers CNS. 2020. PMID: 32650790 Free PMC article. Review.
-
Persistent Neurovascular Unit Dysfunction: Pathophysiological Substrate and Trigger for Late-Onset Neurodegeneration After Traumatic Brain Injury.Front Neurosci. 2020 Jun 9;14:581. doi: 10.3389/fnins.2020.00581. eCollection 2020. Front Neurosci. 2020. PMID: 32581697 Free PMC article.
Publication types
MeSH terms
Grant support
LinkOut - more resources
-
Full Text Sources
-
Other Literature Sources
-
Medical
