- Journal List
- Wiley-Blackwell Online Open
- PMC6590171
Impact of Free‐Choice Diets High in Fat and Different Sugars on Metabolic Outcome and Anxiety‐Like Behavior in Rats
 1
1
Fiona Peris‐Sampedro
1 Department of Physiology/Endocrine, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden,
Myriam Mounib
1 Department of Physiology/Endocrine, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden,
Erik Schéle
1 Department of Physiology/Endocrine, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden,
Christian E. Edvardsson
1 Department of Physiology/Endocrine, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden,
Iris Stoltenborg
1 Department of Physiology/Endocrine, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden,
Roger A. H. Adan
1 Department of Physiology/Endocrine, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden,
2 Brain Center Rudolf Magnus, Department of Translational Neuroscience, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands,
Suzanne L. Dickson
1 Department of Physiology/Endocrine, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Gothenburg, Sweden,
 Corresponding author.
Corresponding author.Associated Data
Abstract
Objective
Rats were exposed to free‐choice diets (fat plus one of two different sugar solutions, glucose or sucrose), and the metabolic consequences and impact on locomotor activity and anxiety‐like behavior were explored.
Methods
For 3 weeks, 7‐week‐old male rats were offered either chow only or free‐choice high‐fat diets differing in their added sugar: no sugar, sucrose, or glucose. In a second experiment, after 2 weeks on the diets, rats were switched from high sucrose to high glucose for two additional weeks. Metabolic end points included body weight, food intake, food choice, glycemic control, metabolic hormones, fat pad weight, brown adipose tissue weight, and gene expression. Behavioral analysis included locomotor and anxiety‐like activity in the open field and elevated plus maze.
Results
Both sugar diets enhanced adiposity and induced hyperphagia, favoring unhealthier dietary selection above that of the control diets (chow or free‐choice high‐fat with no sugar). Despite isocaloric intake in the sugar‐containing diets, offering glucose instead of sucrose was associated with improved insulin sensitivity. The sugar‐containing diets reduced activity (but with movements of increased velocity) and induced an anxiety‐like phenotype.
Conclusions
Although free‐choice diets negatively impacted on metabolism and anxiety‐like behavior, replacing sucrose with glucose improved insulin sensitivity and may therefore be better for health.
Introduction
Obesity is caused by a complex interplay between behavioral, genetic, physiological, social, economic, and environmental factors such as diet and lifestyle. Evidence is emerging that the quality of the diet may be as (or even more) important as its caloric content for body weight outcomes 1. Dietary fat has been considered the main culprit for the escalating obesity pandemic, with added sugars as a secondary concern 2. This dogma, which prevailed for many decades, is now being challenged as many epidemiological studies, as well as mechanistic studies in rodents, have highlighted the importance of increased consumption of added sugars, particularly in sugar‐sweetened beverages, in obesity development and associated metabolic diseases, including type 2 diabetes 2, 3, 4. Although still controversial, the sugar content/composition of the diet may also be of relevance for behaviors expressed in certain neuropsychiatric diseases involving hyperactivity and/or anxiety 5, 6.
Sucrose, a disaccharide composed of glucose and fructose, remains the primary added sweetener worldwide. Although both monosaccharides are hexoses, their metabolism is considerably different 3, 7. Fructose is glycogenic (although less than glucose) but, in contrast to glucose, also has lipogenic properties 7, 8. The sweetness of fructose is approximately twice as high as glucose. The dietary paradigms used in rodents to explore these properties largely have provided sugar in liquid form alone (with liquid glucose rarely explored) or in combination with other nutrients in a single solid pellet (such that the animals cannot select one nutrient over another). To better mimic human daily consumption in Western‐style diets, La Fleur and coworkers 9, 10, 11 validated an obesogenic and hyperphagic free‐choice rodent paradigm, the so‐called free‐choice high‐fat high‐sucrose (fcHFHS) diet, in which the highly caloric components of the diet (i.e., liquid sucrose and fat) are offered separately along with a nutritionally balanced chow diet. Overall, rats fed this diet persistently overconsume, which ultimately results in increased adiposity and insulin resistance 9, 10, 11, 12.
The present study sought to determine whether the metabolic consequences of offering free‐choice diets to rats differ depending on the sugar solution offered (sucrose vs. glucose). We reasoned that because glucose levels in blood are sensed and tightly regulated, providing glucose rather than sucrose would result in induction of satiation, which would limit consumption and accumulation of fat. To this end, we explored adiposity and metabolic parameters in two studies, one that directly compared the impact of a fcHFHS diet with a free‐choice high‐fat high‐glucose (fcHFHG) diet over 3 weeks and another in which, after 2 weeks, the diet was switched from fcHFHS to fcHFHG for 2 more weeks. Finally, given the need for a better understanding between dietary sugars and mental health, we sought to determine whether rats fed these free‐choice high‐fat high‐sugar diets differ in their locomotor and/or anxiety‐like behavior.
Methods
Animals and care
Two different sets of adult male Sprague‐Dawley rats (7 weeks old; Charles River, Sulzfeld, Germany) were used. They were individually housed under a 12‐hour light/dark cycle (lights off: 7:00 pm) in standard controlled conditions (20‐22°C and 50% humidity) and were allowed to acclimate for 1 week with free access to standard maintenance chow (Teklad diet 2016; Harlan Laboratories, Cambridgeshire, UK; 3.00 kcal/g: 22% protein, 66% carbohydrate, 12% fat). They were then switched to their experimental diets until the end of the experiments. Water was available ad libitum at all times. Animal procedures were approved by the local ethics committee for animal care in Gothenburg, Sweden (Göteborgs djurförsöksetiska nämnd; 28–2015) and were conducted in compliance with guidelines.
Diet intervention procedures
In the first experiment (315.7 ± 3.2 g body weight), rats were divided into four groups balanced in body weight and food intake: the control group (n = 8), which remained on ad libitum regular chow throughout the whole experiment; the fcHF group (n = 10), which had free access to lard (saturated animal fat; Dragsbæk, Thisted, Denmark; 9.00 kcal/g: 100% fat) and regular chow; the fcHFHS group (n = 10), which was offered continuous access to lard, a 9% sucrose solution (Dansukker, Malmö, Sweden; 0.36 kcal/mL: 100% carbohydrate), and regular chow; and the fcHFHG group (n = 10), which differed from the fcHFHS group only in the substitution of sucrose with a 9% glucose solution (Now Real Food, Bloomingdale, Illinois; 0.36 kcal/mL: 100% carbohydrate). Rats were maintained on the diets for 3 weeks.
Baseline sugar preference was tested before starting the dietary intervention. After familiarization of the two‐bottle setup (filled with water) over 2 days, rats were presented with two bottles (9% sucrose and 9% glucose) for 3 hours per day on three consecutive days. The two bottles were switched each day. Preference for each sweet solution was calculated as follows: percent sucrose or glucose preference = (milliliters of sucrose or glucose ÷ total liquid intake) × 100.
In the second experiment (243.3 ± 2.0 g body weight), rats were first divided into two balanced groups: the control group (n = 14) and the fcHFHS group (n = 30). After 2 weeks, fcHFHS‐fed rats were either kept on the same diet (n = 11) or switched to a fcHFHG diet (n = 11) instead for 2 more weeks.
We chose a 9% sugar solution because this concentration matches the range of calories per milliliter in commercially available sugar‐sweetened beverages. All animals were weighed three times per week. Food and liquid intake were recorded daily (24 hours).
Behavioral assessment
We sought to better quantify our observation that sugar‐exposed rats seemed more “jumpy” or anxious. Rats were first handled daily for 3 days. Tests were conducted during the light phase and groups counterbalanced regarding the time of testing. To evaluate general locomotor activity and anxiety‐like behavior, rats from the second experiment were tested in an open field (OF) and an elevated plus maze (EPM), respectively, at the end of the experiment. The path and movements in the OF were computerized by means of Activity Monitor 7 tracking software (Med Associates Inc., St. Albans, Vermont), and the activity in the EPM was controlled using a Fader Control interface and Med‐PC IV software (Med Associates).
OF
The OF consisted of a bright‐white 90 × 90‐cm arena, protected with 30‐cm‐high walls (Med Associates) and divided into two zones: the periphery and the central area (45 × 45 cm). Each rat was initially placed in the left corner of the OF and allowed to freely explore for 30 minutes. We measured the total distance traveled, time ambulatory, average velocity, vertical activity, stereotypic activity, and time immobile (defined as time spent with no new beam breaks). To study anxiety‐like behavior, we analyzed activity in the central area (i.e., the ratio of total distance in the center to total distance, time spent in the center, frequency of visits to the center, and time immobile in the center and in the periphery).
EPM
The EPM (Med Associates) consisted of two open arms elevated 70 cm above the ground (50 × 10 cm), crossed by two closed arms (50 × 10 cm) with 40‐cm‐high protective walls. A neutral central platform (10 × 10 cm) connected the four arms. Each rat was initially placed in the junction area facing a closed arm and allowed to freely explore for 5 minutes. We determined the percentage of entries into the open arms and the time spent in the open and closed arms.
Euthanization and sampling
All rats from the first experiment were euthanized at the end of the 3‐week dietary intervention. Regarding the second experiment, a first euthanization occurred at the end of the second week of exposure (chow, n = 6; fcHFHS, n = 8), whereas all the remaining rats (chow, n = 8; fcHFHS, n = 11; fcHFHG, n = 11) were euthanized by the end of the 4‐week dietary exposure. Rats were not given access to lard and sugar from 6:00 pm on the day prior to euthanization to avoid any potential interference of circulating lipids and glucose on the parameters measured 12. All rats were anesthetized with isoflurane prior to decapitation, and trunk blood was collected. Serum samples were stored at −80°C for subsequent analysis of hormones. Interscapular brown adipose tissue (BAT) was rapidly dissected, weighed, snap frozen in liquid nitrogen, and stored at −80°C for later determination of gene expression. All euthanizations were carried out between 9:00 am and 2:00 pm.
Body composition and leptin measurement
For each rat, epididymal, subcutaneous (inguinal), and perirenal with retroperitoneal white adipose tissues were dissected and weighed. Serum leptin was measured in duplicate (ELISA kit #EZRL‐83K; Merck Millipore, Darmstadt, Germany), according to the manufacturer’s instructions.
Insulin sensitivity assessment
Blood glucose was determined using a glucometer (Roche Diagnostics Scandinavia AB, Bromma, Sweden). Serum insulin was determined in duplicate (ELISA kit #EZRMI‐13K; Merck Millipore). Insulin sensitivity was assessed using the homeostatic model assessment of insulin resistance (HOMA‐IR) 13. HOMA‐IR is equal to fasting glucose (millimoles per liter) multiplied by fasting insulin (milliunits per liter) divided by 22.5 14, 15.
RNA extraction and complementary DNA synthesis
Total RNA from BAT samples was extracted with a RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Genomic DNA was removed by DNase treatment (Qiagen). RNA abundance and purity were assessed spectrophotometrically with NanoDrop technology (Thermo Fisher Scientific, Inc., Wilmington, Delaware). Complementary DNA (cDNA) was synthesized from 500 ng of total RNA using an iScript cDNA Synthesis Kit (Bio‐Rad Laboratories, Hercules, California).
Quantitative real‐time polymerase chain reaction analysis
A 10‐µL quantitative real‐time polymerase chain reaction (PCR) was performed with a TaqMan Fast Advanced Master Mix kit (Applied Biosystems, Foster City, California). Thermal cycling and fluorescence detection were carried out with QuantStudio 7 Flex Real‐Time PCR System (Applied Biosystems). Data were analyzed with QuantStudio Real‐Time PCR software (version 13; Applied Biosystems), which set the cycle threshold (Ct) automatically. Relative changes in transcript levels were normalized to the housekeeping gene hydroxymethylbilane synthase (Hmbs) according to the 2−ΔCt method. Primers are listed in Supporting Information Table S1.
Statistics
Data were processed using SPSS software 24 (IBM Corp., Armonk, New York). Repeated‐measures analysis of variance (ANOVA) was used for comparing continuous variables, with Diet as the between‐subject factor and Time (weeks) as the within‐subject factor. One‐way ANOVA (Diet) was used when comparing two or more groups. Tukey post hoc tests for multiple comparisons were used to follow up significances and interactions. Pearson correlations were performed to assess linear associations between two variables. We used a paired t test to study sugar preference. Statistical significance was set at P F, P, and r values, are given in figures or figure legends.
Results
Body weight, body composition, and leptin levels
Free access to saturated fat and sucrose or glucose solutions superimposed upon regular chow led to an obesity‐like phenotype (Figure (Figure1).1). The increase in body weight, which was similar between both fcHFHS and fcHFHG groups, was only observed in the first experiment (Figure (Figure1A‐1B).1A‐1B). However, fat mass was enhanced overall in both sugar‐exposed groups (either fcHFHS or fcHFHG) but not in the fcHF group (Figure (Figure11C‐1D). Specifically, in the first experiment (Figure (Figure1,1, upper panels), the weight of all dissected fat depots increased similarly after 3 weeks on either fcHFHS or fcHFHG diets compared with the control group (Figure (Figure1C).1C). Likewise, in the second experiment (Figure (Figure1,1, lower panels), both perirenal white adipose tissue and total white adipose tissue were already increased in fcHFHS‐fed rats relative to the chow group at 2 weeks on the diet. The effect of dietary intervention on adiposity was even greater by the end of the 4‐week exposure period and was indistinguishable between both sugar groups (Figure (Figure1D).1D). Accordingly, leptin levels, reflecting fat mass, were solely (and similarly) increased in fcHFHS and fcHFHG groups compared with the control groups in both experiments (Figure (Figure11E‐1F). Replacing sucrose with glucose did not cause leptin levels to differ (Figure (Figure11F).
Consumption of either free‐choice high‐fat high‐sucrose (fcHFHS) or free‐choice high‐fat high‐glucose (fcHFHG) diets leads to an overall similar obesity phenotype in two different experiments. (A) Body weight (BW) gain was significantly increased in rats on both fcHFHS and fcHFHG diets compared with the control group after 3 weeks of exposure (Diet: [F(3, 37) = 8.735, P 0.001]). Free‐choice high‐fat (fcHF)‐fed rats showed similar BW gain to chow‐fed rats. (B) BW gain remained unchanged in the second experiment. (C) Epididymal, subcutaneous, and perirenal with retroperitoneal white adipose tissue (eWAT, sWAT, and pWAT, respectively), as well as total WAT (tWAT) per 100 g of BW, was significantly and similarly increased in both fcHFHS and fcHFHG groups compared with their control peers after 3 weeks of exposure (Diet for: eWAT: [F(3, 37) = 4.432, P = 0.010]; sWAT: [F(3, 37) = 5.868, P = 0.002]; pWAT: [F(3, 37) = 7.327, P = 0.001]; tWAT/100 g BW: [F(3, 37) = 6.485, P = 0.001]). (D) In the second experiment, fcHFHS‐fed rats exhibited higher pWAT (Diet: [F(1, 13) = 6.273, P = 0.028]) and tWAT/100 g BW (Diet: [F(1, 13) = 6.714, P = 0.024]) than chow‐fed rats after 2 weeks on the diet. After 4 weeks, the effect of the dietary intervention was even greater (Diet for: sWAT: [F(2, 29) = 4.190, P = 0.026]; pWAT: [F(2, 29) = 3.861, P = 0.034]; tWAT/100 g BW: [F(2, 29) = 4.947 P = 0.015]) but indistinguishable between both sugar groups. (E) In line with the fat weight data, leptin levels were exclusively increased in both fcHFHS and fcHFHG groups relative to the control group in the first experiment (Diet: [F(3, 37) = 3.702, P = 0.021]). (F) In the second experiment, leptin levels were significantly higher in fcHFHS‐fed rats than in chow‐fed rats after 2 weeks on the diet (Diet: [F(1, 13) = 7.354, P = 0.019]). By the end of the 4‐week exposure period, both sugar groups exhibited a similar increase in leptin levels compared with the control group (Diet: [F(2, 29) = 5.936, P = 0.007]). Upper panels: first experiment; lower panels: second experiment. Data were analyzed using one‐way ANOVA and are shown as means ± SEM. Asterisks indicate significant differences for that group compared with the corresponding control group according to Tukey post hoc tests: *P 0.05; **P 0.01; and ***P 0.001.
Caloric intake and dietary choice
Adding free access to saturated fat with or without sugar solutions to regular chow caused hypercaloric consumption (Figure (Figure2).2). In the first experiment (Figure (Figure2,2, upper panels), fcHF‐, fcHFHS‐, and fcHFHG‐fed rats consumed more calories daily than chow‐fed rats throughout the 3‐week exposure, with both fcHFHS and fcHFHG groups consuming the most (Figure (Figure2A).2A). Indeed, the more choices offered, the greater the hyperphagic effect observed (Figure (Figure2A‐2B).2A‐2B). Similarly, in the second experiment (Figure (Figure2,2, lower panels), fcHFHS‐fed rats ate more calories daily than the control group during the first 2 weeks (Figure (Figure2C).2C). Hyperphagia persisted until the end of the 4 weeks, and we did not observe differences in total caloric intake between the sugar groups after replacing sucrose with glucose in the diet (Figure (Figure22C‐2D).
Hyperphagia emerges as a result of exposure to free‐choice diets. (A) Repeated‐measures ANOVA (RMANOVA) revealed significant differences of Diet (F[3, 37] = 39.163, P 0.001) and Time (F[3, 37] = 114.732, P 0.001), as well as the interaction of these two factors (F[9, 37] = 5.706, P 0.001) on daily caloric intake throughout the 3‐week exposure. Both free‐choice high‐fat high‐sucrose (fcHFHS) and free‐choice high‐fat high‐glucose (fcHFHG) groups consumed more calories daily than both the control and the free‐choice high‐fat (fcHF) groups, whereas fcHF‐fed rats exhibited higher daily caloric intake than chow‐fed rats. (B) Consistently, total caloric intake was increased over the 3‐week period (one‐way ANOVA, Diet: [F(3, 37) = 46.445, P = 0.001]). Specifically, all the free‐choice groups consumed more calories than the control group, while both fcHFHS and fcHFHG groups also displayed increased total caloric intake compared with the fcHF group. (C) RMANOVA detected an upward general effect of Diet (F[1, 43] = 59.687, P 0.001), as well as an effect of Time (F[2, 43] = 172.793, P 0.001) and of the interaction of these two factors (F[2, 43] = 75.627, P 0.001), on daily caloric intake over the first 2 weeks following introduction of the diet. After sucrose replacement by glucose, daily consumption remained steady within groups; both fcHFHS and fcHFHG groups indistinctly continued to consume more calories daily than the control group until the end of the diet exposure (RMANOVA, Diet: [F(2, 29) = 17.472, P 0.001]). (D) Accordingly, total caloric intake over the 4‐week period was increased in both the fcHFHS and fcHFHG group compared with the control group (one‐way ANOVA, Diet: [F(2, 29) = 22.107, P 0.001]). Upper panels: first experiment; lower panels: second experiment. Data are shown as means ± SEM. Symbols indicate significant differences according to Tukey post hoc tests: versus the chow group at **P 0.01 and ***P 0.001; versus the fcHF group at †† P 0.01 and ††† P 0.001.
Regardless of the experiment, all rats fed the free‐choice diets compensated for increased lard and/or sugar consumption by reducing their chow intake (Figure (Figure3A‐3B).3A‐3B). Lard intake was similar between all free‐choice groups in both experiments (Figure (Figure3C‐3D).3C‐3D). Baseline two‐bottle sugar‐preference testing, conducted prior to starting the first experiment, revealed that rats markedly preferred sucrose (65.64% ± 1.64%) to glucose (34.36% ± 1.64%) (t[37] = 9.516; P 0.001]. Accordingly, results from the first experiment reveal that fcHFHS rats drank more sugar than fcHFHG rats (Figure (Figure3E),3E), although this did not affect total caloric intake, body weight, or adiposity. Interestingly, fcHFHG rats previously exposed to a sucrose solution for 2 weeks showed similar total sugar intake to fcHFHS rats at the end of the second experiment (Figure (Figure33F).
Dietary choice upon exposure to free‐choice diets. (A) In the first experiment, all the free‐choice diet groups (free‐choice high‐fat [fcHF]; free‐choice high‐fat high‐sucrose [fcHFHS]; free‐choice high‐fat high‐glucose [fcHFHG]) reduced their chow intake overall compared with the control group (Diet: [F(3, 37) = 16.498, P 0.001]). (B) Similarly, fcHFHS‐ and fcHFHG‐fed rats from the second experiment ate less chow than the control group after 4 weeks on their respective diets (Diet: [F(2, 29) = 13.686, P 0.001]). (C,D) Total lard intake was similar between all the free‐choice diet groups in both experiments. (E) In the first experiment, the 3‐week total intake of sugar was significantly higher in fcHFHS‐fed than in fcHFHG‐fed rats (Diet: [F(1, 19) = 8.079, P = 0.011]). (F) fcHFHG rats previously exposed to a sucrose solution for 2 weeks showed similar total sugar intake to fcHFHS rats after the 4‐week dietary intervention. Upper panels: first experiment; lower panels: second experiment. Data were analyzed using one‐way ANOVA and are shown as means ± SEM. Symbols indicate significant differences according to Tukey post hoc tests: versus the chow group at **P 0.01 and ***P 0.001; versus the fcHFHS group at #P 0.05.
Insulin sensitivity
Although blood glucose did not differ between rats fed any of the free‐choice diets (Figure (Figure4A‐4B),4A‐4B), there were diet‐dependent differences in insulin levels in both experiments. In experiment 1 (Figure (Figure4,4, upper panels), fcHFHS rats, but not fcHFHG rats, displayed increased insulin levels after 3 weeks on the diet compared with chow‐fed controls (Figure (Figure4C).4C). Similarly, in experiment 2 (Figure (Figure4,4, lower panels), serum insulin was increased in the fcHFHS diet relative to the chow group after 2 and 4 weeks (Figure (Figure4D).4D). Strikingly, replacing sucrose with glucose in the paradigm improved this effect: fcHFHG‐fed rats exhibited similar insulin levels to chow‐fed rats at the end of the 4‐week dietary intervention (Figure (Figure4D).4D). Consistent with this, only the fcHFHS rats had increased rates of insulin resistance, reflected by enhanced HOMA‐IR index scores relative to the control group in both experiments (Figure (Figure44E‐4F).
Differential effects of sucrose and glucose (offered together with fat and chow) on circulating insulin levels and insulin sensitivity. (A,B) Blood glucose levels remained similar between all the free‐choice diets (free‐choice high‐fat [fcHF]; free‐choice high‐fat high‐sucrose [fcHFHS]; free‐choice high‐fat high‐glucose [fcHFHG]) and their respective control groups in both experiments. (C) Serum insulin was increased only in fcHFHS‐fed rats compared with chow‐fed rats when assessed after 3 weeks of dietary exposure (Diet: [F(3, 37) = 5.458, P = 0.004]). (D) Similarly, only fcHFHS diet led to a significant increase in serum insulin levels after 2 weeks (Diet: [F(1, 13) = 7.282, P = 0.019]) and 4 weeks (Diet: [F(2, 29) = 5.049, P = 0.014]). (E) Accordingly, only rats fed fcHFHS for 3 weeks had increased homeostatic model assessment of insulin resistance (HOMA‐IR) scores compared with the chow group (Diet: [F(3, 37) = 5.019, P = 0.005]). (F) Likewise, HOMA‐IR scores were enhanced after 2 weeks (Diet: [F(1, 13) = 7.325, P = 0.019]) and 4 weeks (Diet: [F(2, 29) = 4.187, P = 0.026]) on the fcHFHS diet. Rats switched to the fcHFHG diet showed similar HOMA‐IR scores as the chow‐fed rats (Tukey post hoc test, P = 0.256). Upper panels: first experiment; lower panels: second experiment. Data were analyzed using one‐way ANOVA and are shown as means ± SEM. Asterisks indicate significant differences for that group compared with the corresponding control group according to Tukey post hoc tests: *P 0.05 and **P 0.01.
Expression of BAT‐related genes
BAT weight was higher in both fcHFHS and fcHFHG groups than in the chow group elsewhere (Figure (Figure5A‐5B)5A‐5B) and higher in fcHFHS‐fed rats than in fcHF‐fed rats in the first experiment (Figure (Figure5A).5A). When examining the expression of two classical BAT genes reflecting its metabolic activity (uncoupling protein‐1 [Ucp‐1]; cell death‐inducing DNA fragmentation factor alpha‐subunit‐like effector a [Cidea]), we were unable to detect significant differences in expression between groups in either experiment (Figure (Figure5C‐5D).5C‐5D). Remarkably, expression of peroxisome proliferator‐activated receptor gamma coactivator 1 alpha (Pgc‐1α; also known as Ppargc1a), involved in mitochondrial biogenesis, was significantly reduced after consuming either fcHFHS or fcHFHG diets compared with the control group in experiment 1, while tended to be in experiment 2 (Figure (Figure5C‐5D,5C‐5D, Supporting Information Figure S1). Expression of transcription factor forkhead box C2(Foxc2), which has been linked to a lean and insulin‐sensitive phenotype, tended to increase in fcHFHG‐fed rats compared with fcHF‐fed rats in experiment 1 (Figure (Figure55C).
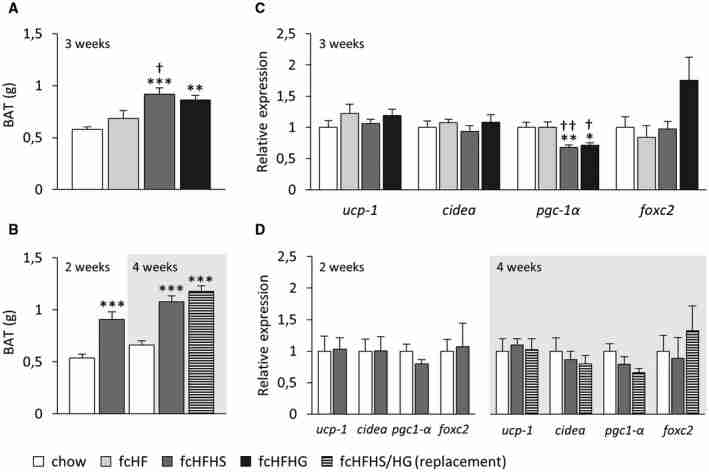
Expression of classical brown adipose tissue (BAT) genes and genes linked to mitochondrial biogenesis and function after exposure to free‐choice diets. (A) Compared with the control group, the interscapular BAT depot was significantly increased in weight after exposure to free‐choice high‐fat high‐sucrose (fcHFHS) and free‐choice high‐fat high‐glucose (fcHFHG) diets. Moreover, fcHFHS‐fed rats also exhibited increased BAT weight compared with the free‐choice high‐fat (fcHF) group (Diet: [F(3, 37) = 9.047, P 0.001]). (B) In the second experiment, fcHFHS‐fed rats exhibited higher BAT than chow‐fed rats after 2 weeks on the diet (Diet: [F(1, 13) = 17.685, P = 0.001]). After replacing sucrose with glucose, the effect remained steady and indistinguishable between both sugar groups (Diet: [F(2, 29) = 22.954, P 0.001]). (C) In the first experiment, expression of peroxisome proliferator‐activated receptor gamma coactivator 1 alpha (Pgc‐1α) was significantly reduced after exposure to either fcHFHS or fcHFHG diets compared with the control and the fcHF groups (Diet: [F(3, 37) = 7.517, P = 0.001]). We also detected a significant Diet effect (F[3, 37] = 3.007, P = 0.044) on the expression of transcription factor forkhead box C2 (Foxc2); Foxc2 tended to be expressed at higher levels in fcHFHG‐fed rats compared with fcHF‐fed rats (P = 0.063). (D) In the second experiment, Pgc‐1α expression tended to be different overall by the end of the 4‐week dietary exposure (Diet: [F(2, 29) = 2.702, P = 0.085]), and results followed the same trend. Neither the dietary intervention nor the sucrose replacement by glucose altered the expression of the other BAT‐related genes. Upper panels: first experiment; lower panels: second experiment. Data were analyzed using one‐way ANOVA and are shown as means ± SEM. Symbols indicate significant differences according to Tukey post hoc tests: versus the corresponding control group at *P 0.05; **P 0.01; and ***P 0.001; versus the fcHF group at † P 0.05 and †† P 0.01.
General locomotor activity and anxiety‐like behavior
Because rats exposed to the free‐choice high‐sugar diets in the first experiment appeared to be more “jumpy,” in the second experiment, we explored anxiety and locomotor activity. Although we found significant behavioral effects compared to the control group, fcHFHS and fcHFHG rats behaved similarly (Supporting Information Table S2), and so we combined them into one high‐fat high‐sugar group for further analysis.
Rats exposed to diets rich in fat and different sugars traveled significantly less distance in the OF (Figure (Figure6A),6A), spent less time ambulating (Figure (Figure6B),6B), reduced their stereotypic behavior (Figure (Figure6C),6C), and spent more time immobile (Figure (Figure6D)6D) compared with chow controls. Nonetheless, when they did move, their movements were faster than the controls (Figure (Figure6E).6E). The dietary intervention did not affect vertical activity in the OF (Figure (Figure66F).
Exposure to mixed palatable free‐choice diets induces hypoactivity while simultaneously increasing velocity. (A) Rats exposed to free‐choice diets (free‐choice high‐fat high‐sucrose [fcHFHS]; free‐choice high‐fat high‐glucose [fcHFHG]) traveled significantly less distance in the open field (OF) (Diet: F[1, 29] = 6.536, P = 0.016), (B) spent less time ambulating (Diet: F[1, 29] = 10.820, P = 0.003), (C) reduced their stereotypic behavior (Diet: F[1, 29] = 7.171, P = 0.012), and (D) spent more time immobile (Diet: F[1, 29] = 5.452, P = 0.027) compared with chow‐fed rats. (E) Average velocity was increased in the free‐choice group compared with the control group (Diet: F[1, 29] = 39.782, P 0.001). (F) Dietary intervention did not affect vertical activity in the OF. Data were analyzed using one‐way ANOVA and are shown as means ± SEM. Asterisks indicate significant differences for that group compared with the control group: *P 0.05; **P 0.01; and ***P 0.001.
As for anxiety measures, rats on the high‐fat high‐sugar diets spent less time in the center of the OF (Figure (Figure7A)7A) and more time motionless in the periphery compared with chow controls (Figure (Figure7B).7B). Accordingly, they also tended to spend less time resting in the central area of the field (Figure (Figure7C).7C). The dietary intervention did not affect the frequency of visits to the central area nor the ratio of distances (Figure (Figure7D‐7E).7D‐7E). Although not significant, the results in the EPM are in line with those of the OF, pointing to the establishment of a phenotype resembling anxiety after exposure to mixed palatable free‐choice diets. Specifically, exposed rats tended to spend less time in the open arms (Figure (Figure7F)7F) but longer in the closed arms relative to their controls (Figure (Figure7G).7G). The percentage of entries into the open arms was similar between the intervention and the control group (Figure (Figure77H).
Exposure to mixed palatable free‐choice diets promotes the emergence of a phenotype resembling anxiety. (A) Rats from the free‐choice group (free‐choice high‐fat high‐sucrose [fcHFHS]; free‐choice high‐fat high‐glucose [fcHFHG]) spent less time in the center of the open field (OF) (Diet: F[1, 29] = 4.392, P = 0.045), (B) while also remaining motionless longer in the periphery compared with chow‐fed rats (Diet: F[1, 29] = 5.972, P = 0.021). (C) We found a downward trend in the time the exposed rats spent resting in the central area of the field compared with the control group (Diet: F[1, 29] = 3.979, P = 0.056). (D,E) The dietary intervention did not affect the frequency of visits to the central area of the field or the ratio of distances in the OF. (F,G) Exposed rats tended to spend less time in the open arms (Diet: F[1, 29] = 3.945, P = 0.057) but rather longer in the closed arms of the elevated plus maze (EPM) than their control peers did (Diet: F[1, 29] = 2.990, P = 0.095). (H) Percentage of entries into the open arms of the EPM was similar between the intervention and the control group. Data were analyzed using one‐way ANOVA and are shown as means ± SEM. Asterisks indicate significant differences for that group compared with the control group: *P 0.05.
In line with the behavioral results, most of the parameters studied in the OF (Figure (Figure8A)8A) and the EPM (Figure (Figure8B)8B) correlated with the total 4‐week caloric intake (see also Supporting Information Figure S2), the total sugar intake (Supporting Information Figure S3), and body weight (Supporting Information Figure S4).
Behavioral parameters assessed in the open field (OF) and the elevated plus maze (EPM) correlate with the 4‐week total caloric intake. The total 4‐week caloric intake shown in relationship to (A) several activity parameters in the OF (from left to right: total distance traveled, time ambulatory, time immobile, and average velocity) and (B) anxiety‐like measures in both the OF and EPM (from left to right: time spent in the center of the OF, time spent in the open arms of the EPM, time spent in the closed arms of the EPM, and percentage of entries into the open arms of the EPM). Pearson correlations were performed to assess linear associations between two variables. Asterisks indicate significant Pearson correlation coefficient: *P 0.05; **P 0.01; and ***P 0.001.
Discussion
Here, we explored whether the type of sugar combined with fat and chow in a free‐choice paradigm 9, 10, 11 is important for the metabolic outcome and/or for the expression of anxiety‐like behaviors. We found that, regardless of the sugar offered, sucrose or glucose, rats exposed to these free‐choice diets (fcHFHS and fcHFHG, respectively) rapidly developed an obesity‐like phenotype, reflected by enlarged white adipose tissue and BAT depots, increased leptin levels, and hyperphagia. The type of sugar offered did impact, however, on glycemic control because only rats on the fcHFHS diet exhibited increased insulin levels and HOMA‐IR scores. Strikingly, switching from sucrose to glucose mid experiment was enough to improve the incipient sucrose‐dependent impairment of insulin sensitivity. In line with this, the ingestion of glucose, but not sucrose, was associated with a mild increase in Foxc2 in BAT, a candidate gene for counteracting diet‐induced insulin resistance. Interestingly, enhanced expression of Foxc2 in BAT results in a lean and insulin‐sensitive phenotype 16, 17. The consumption of both sugars in the free‐choice diets led to reduced expression of Pgc‐1α in BAT, indicative of impaired mitochondrial biogenesis. Finally, we found that exposure to mixed palatable free‐choice diets impacted upon behavior, inducing hypoactivity (but increasing velocity) and prompting a phenotype resembling anxiety.
Free‐choice diets such as those used here are more effective in promoting fat accumulation than diets of similar composition given in a single (blended) pellet 9, 10, 11. Although our data match those previously reported 9, 10, 11, an increased weight gain was only observed in the first experiment. This result is perhaps not surprising because rats, and specifically the Sprague‐Dawley strain, are resistant to diet‐induced obesity 18, 19. However, we do not exclude the possibility that the initial body weight (which was lower in the second set of rats than in the first one) and/or the concentration of the sugar used (9% used here because it resembles the levels found in sugar‐sweetened beverages rather than the 30% used in other studies) 9, 10, 11 may have contributed to such differences.
Our data resonate with studies showing that offering free access to palatable diets causes overconsumption 9, 10, 11; the more foods available to choose from, the more calories were consumed, and food choice changed in a manner favoring less intake of healthier chow. Interestingly, our data reveal that this hyperphagic effect is independent of the sugar consumed because rats from both sugar groups balanced their nutrient consumption to eventually reach a similar total caloric intake. Rats find glucose less palatable than sucrose 20, resembling the perception of higher sweetness of sucrose compared with glucose in humans, and therefore it is reasonable to expect that fcHFHG‐fed rats would consume fewer calories from sugar than fcHFHS‐fed rats, and this was what we observed in the first experiment. Interestingly, when previously exposed to a sucrose solution for 2 weeks, rats switched to the fcHFHG diet mid experiment consumed a similar number of calories in the form of sugar to the rats offered the fcHFHS diet only. This result suggests that a previous experience with sucrose might be enough to suppress the initial taste preference, thus implying that the nature of the sugar consumed from then on is irrelevant for maintaining a hyperphagic status.
Recent studies have highlighted the importance of including fat as one of the choices in the free‐choice diets for the metabolic complications; although free access to either liquid fructose, glucose, or sucrose alone increases adiposity in both rats and mice, overconsumption of sugar itself does not lead to insulin resistance 4, 12. Softic et al. reported that mice fed a high‐fat diet supplemented with a 30% fructose solution developed glucose intolerance and had impaired insulin signaling compared with mice fed the same diet but supplemented instead with equal amounts of glucose 4. They reported that, in the setting of a high‐fat diet, fructose supplementation was associated with increased hepatic fatty acid synthesis and marked insulin resistance, while glucose supplementation led to improved insulin signaling, despite having a similar degree of hepatic lipid accumulation. Consistent with these results, we demonstrated that only rats on the fcHFHS diet displayed increased insulin levels and HOMA‐IR index scores. Moreover, our study switching sugars mid experiment indicate that replacing sucrose with glucose has the potential to improve the insulin‐related complications induced by sucrose. It should be noted that both sugar groups exhibited similar weight gain (that in each case was different from the control group) throughout both experiments. Therefore, the changes we observed in insulin levels and HOMA‐IR scores might partially reflect differences in body fat.
In basal conditions, brown adipocytes have the ability to release energy as heat in a process mediated by UCP‐1 21. Recently, it has been suggested that diet‐induced obesity might cause functional hypoxia in BAT ultimately leading to a functional shift from thermogenesis toward lipid storage 22, 23. Our data exploring BAT content and gene expression resonate with previous studies showing that exposure to hypercaloric diets enlarges the interscapular BAT depot 22, 23 and does not alter Ucp‐1 or Cidea expression 23, 24 but reduces the expression of Pgc‐1α 24, suggesting an impaired mitochondrial function upon caloric overload. As previously stated, there is consensus about the potential of Foxc2 in counteracting diet‐induced insulin resistance 16, 17. To the best of our knowledge, the glucose‐dependent effects on Foxc2 upregulation, although inconclusive, have not been reported previously.
The belief that sugar can induce hyperactivity in humans (and rodents) has been a controversial and disputed subject because few studies have successfully established a link between high sugar intake and increased activity in children 6 and experimental animals 25. On the other hand, some authors have suggested that diets rich in fat are anxiolytic 26, 27, but controversy remains 28, 29. Even if there have been studies addressing the direct anxiogenic properties of sugar in the diet 25, most have focused on the effects of its subsequent withdrawal. Here, we demonstrate that a 4‐week exposure to mixed palatable diets decreases general locomotor activity while simultaneously increasing the average velocity. Indeed, rats become more “jumpy” or erratic in their movements, behaviors that resemble agitation and are anxiety‐like 30. Rats from the free‐choice diets spent more time immobile in the OF than chow‐fed controls. Increased immobility in a short OF session (i.e., 30 minutes) likely reflects fear‐induced freezing 30, 31 because it is improbable that animals become habituated to the arena within that short period. Additionally, the increased average velocity observed in rats fed mixed palatable diets could reflect escape behavior, an animal’s innate response upon exposure to panic‐provoking stimulus 32.
Collectively, our data demonstrate that sucrose and glucose (when offered as a free choice together with fat and chow) are similarly obesogenic and that free access to either prompts overconsumption of calories. We also demonstrate that, despite isocaloric intake, glucose consumption does not impact negatively on insulin sensitivity in the setting of a free‐choice diet. Finally, our data exploring the behavioral effects of mixed palatable diets suggest that reigning Western‐style diets, rich in fat and sugar, could contribute to anxiety‐ or agitation‐like symptoms of relevance for mental health and neuropsychiatric disease.
Acknowledgments
We thank Dr. Pol Solé‐Navais for statistical advice and Professor Susanne La Fleur for valuable discussion.
Notes
Funding agencies: The research leading to these results has received funding from the European Union’s Seventh Framework programme for research, technological development, and demonstration under grant agreement no. 607310 (Nudge‐it) as well as from the Swedish Research Council for Health (2016‐02195), the Novo Nordisk Foundation (NNF17OC0027206), Hjärnfonden (FO2017‐0180; FO2018‐0262), and the Swedish state under the agreement between the Swedish Government and the county councils in the ALF agreement (ALFGBG‐723681). We also thank European College of Neuropsychopharmacology (ECNP) for supporting the Nutrition Network.
Disclosure: The authors declared no conflict of interest.
Author contributions: The project idea was initiated by SLD and RAHA. FPS, SLD, and RAHA conceived and planned the rat experiments that were conducted by FPS, MM, CEE, and IS. ES carried out gene expression analyses and analyzed data. FPS analyzed data and wrote the first draft of the manuscript. All authors contributed to the final text and had final approval of the submitted and published versions.