- Journal List
- HHS Author Manuscripts
- PMC4775086

Effects of estrogen on breast cancer development: role of estrogen receptor independent mechanisms
Wei Yue
1Department of Medicine, University of Virginia Health System, Charlottesville, Virginia
Ji-Ping Wang
1Department of Medicine, University of Virginia Health System, Charlottesville, Virginia
Yuebai Li
1Department of Medicine, University of Virginia Health System, Charlottesville, Virginia
Ping Fan
1Department of Medicine, University of Virginia Health System, Charlottesville, Virginia
Guijian Liu
1Department of Medicine, University of Virginia Health System, Charlottesville, Virginia
Nan Zhang
1Department of Medicine, University of Virginia Health System, Charlottesville, Virginia
Mark Conaway
2Department of Public Health Sciences, University of Virginia Health System, Charlottesville, Virginia
Hongkun Wang
2Department of Public Health Sciences, University of Virginia Health System, Charlottesville, Virginia
Kenneth S. Korach
3Receptor Biology Section, Laboratory of Reproductive and Developmental Toxicology, National Institutes of Environmental Health Sciences, NIH, Research Triangle Park, North Carolina
Wayne Bocchinfuso
3Receptor Biology Section, Laboratory of Reproductive and Developmental Toxicology, National Institutes of Environmental Health Sciences, NIH, Research Triangle Park, North Carolina
Richard Santen
1Department of Medicine, University of Virginia Health System, Charlottesville, Virginia
Associated Data
Abstract
Development of breast cancer involves genetic factors as well as lifetime exposure to estrogen. The precise molecular mechanisms whereby estrogens influence breast tumor formation are poorly understood. While estrogen receptor α (ERα) is certainly involved, nonreceptor mediated effects of estradiol (E2) may also play an important role in facilitating breast tumor development. A “reductionist” strategy allowed us to examine the role of ERα independent effects of E2 on mammary tumor development in ERα knockout (ERKO) mice bearing the Wnt-1 oncogene. Exogenous E2 “clamped” at early follicular and midluteal phase levels (i.e., 80 and 240 pg/ml) accelerated tumor formation in a dose-related fashion in ERKO/Wnt-1 animals (p = 0.0002). Reduction of endogenous E2 by oophorectomy (p < 0.001) or an aromatase inhibitor (AI) (p = 0.055) in intact ERKO/Wnt-1 animals delayed tumorigenesis as further evidence for an ER-independent effect. The effects of residual ERα or β were not involved since enhancement of tumor formation could not be blocked by the antiestrogen fulvestrant. 17α-OH-E2, a metabolizable but ER-impeded analogue of E2 stimulated tumor development without measurable uterine stimulatory effects. Taken together, our results suggest that ER-independent actions of E2 can influence breast tumor development in concert with ER dependent effects. These observations suggest 1 mechanism whereby AIs, which block E2 synthesis, would be more effective for breast cancer prevention than use of antiestrogens, which only block ER-mediated effects.
It was estimated that approximately 192,000 women would be diagnosed with breast cancer in the United States in 2009 with 40,000 resulting deaths.1 Improved diagnostic and treatment strategies have decreased breast cancer mortality by 25% over the past 2 decades,2–4 but the physical and psychological burdens of surgery, radiotherapy, hormonal- and chemotherapy are substantial. For this reason, breast cancer prevention represents a major focus of current research.5 A greater understanding of the molecular mechanisms of carcinogenesis will be required, however, before development of improved strategies for breast cancer prevention.
Both genetic and hormonal factors have been implicated in the genesis of breast cancer. Genetic factors involve significant mutations in BRCA 1 and 2, CHEK2, TP53, LKB-1 and PTEN in 5–10% of patients and lower risk mutations inferred by identical twin and genome wide association studies in others.6–8 Epidemiologic and experimental data implicate estradiol (E2) as another contributing factor. In various animal models, E2 administration causes and antiestrogens prevent breast cancer.9,10 In women, bilateral oophorectomy before age 35 reduces the lifetime incidence of breast cancer by 75%.11,12 Increased lifetime exposure to estrogens, conferred by early menarche, late menopause, long-term menopausal estrogen therapy, obesity and high circulating E2 levels in pre- and postmenopausal women, are associated with an enhanced incidence of breast cancer.13–16 Data from 2 large studies demonstrated that postmenopausal women in the highest quintile of plasma free E2 experienced at least a 2.58-fold (95% CI 1.76–3.78) higher rate of breast cancer over the ensuing 10 years than those in the lowest quintile.16,17 Blockade of estrogen action with tamoxifen or raloxifene reduces the incidence of breast cancer by 50–75% in high-risk women.5,18,19 Finally, inhibition of E2 synthesis with aromatase inhibitors (AIs) or abrogation of its action with antiestrogens prevents the development of contralateral breast cancer during adjuvant therapy.20,21 Taken together, these data provide compelling evidence that E2 plays a major etiologic role in breast cancer development.
The precise molecular mechanisms whereby E2 influences breast cancer development are not well understood. The most widely accepted theory, supported by extensive experimental evidence,22,23 holds that E2, acting through ERα, stimulates cell proliferation and initiates mutations that occur as a function of errors during DNA replication. The promotional effect of E2 then supports the growth of cells harboring mutations, which then accumulate until cancer ultimately results. Clinical and experimental data also suggest the possibility that receptor independent effects of E2 may be mechanistically involved. In a recent review, Yager and Davidson describe in detail how estrogen metabolites can exert genotoxic effects, which contribute to the development of breast cancer.24 Estrogens are converted to quinone metabolites, which directly bind to DNA and form adducts. Additionally, catechol estrogen metabolites undergo redox cycling with generation of oxygen free radicals, which damage DNA-bound guanine to form 8-OXO-guanine. The quinone-adducts and 8-OXO-guanine bases are unstable and are deleted from the affected DNA segments through a process called “depurination.”24 Error prone DNA repair then results in the formation of mutations at the depurinated sites. Accumulation of these mutations would then contribute to the development of breast cancer.25 As predicted from the “estrogen genotoxic metabolite” hypothesis, a predisposition to breast cancer would be expected in women with combinations of mutations of estrogen metabolizing enzymes, a finding reported by Park et al.26 and Ritchie et al.27 In support of the depurination mechanism, 2 recent reports indicate that women with breast cancer or at high risk for the disease have significantly higher levels of depurinating estrogen-DNA adducts in their urine than women at normal risk for breast cancer.28,29
Cell culture and animal data have provided biochemical and biologic evidence that ER independent DNA damage from E2 occurs.30–32 However, the causal relationship between E2 metabolism and breast cancer development has been a controversial issue. To date, no direct proof of an ER-independent effect of E2 on mammary tumor formation in an animal model has been reported. For this reason, we decided to provide proof of the principle that ER independent effects of estrogen could influence breast cancer development in an in vivo system. We chose the estrogen-receptor knockout (ERKO) mouse model system, which would allow assessment of the effects of E2 acting independently of ERα function. Reasoning that E2 exerts modulating effects on breast cancer incidence in women with genetic defects, we knocked in the Wnt-1 gene in the ERKO mouse to generate a double transgenic mouse model for our studies. This model mimics high-risk patients with genetic defects and provides a system with a sufficiently high frequency of tumor development to make the studies feasible.
We recognized that various factors inherent in our experimental design would confound interpretation of data and therefore attempted to utilize a “reductionist approach,” a term originally introduced by Bernard in 1864.33 Accordingly, to minimize confounding factors inherent in our model, we removed the ovaries in ERα knockout mice to eliminate potential confounding effects of ovarian steroid and peptide hormones and administered exogenous estradiol. We also administrated the pure antiestrogen (fulvestrant) to ensure complete blockade of any residual ERα and ERβ. Using this approach, our data strongly suggest that E2 can influence tumor formation through ER-independent effects.
Material and Methods
Animals
Wnt-1 transgenic animals were obtained from Dr. Harold Varmus and bred at the National Institute of Environmental Health Sciences (NIEHS).34–36 These animals were then crossbred with heterozygous ERα knockout mice to generate Wnt-1 transgenic mice, which could then be further bred to produce ERα knockout/Wnt-1 double transgenics (ERKO/Wnt-1). With establishment of collaboration among investigators, breeding pairs were sent to the University of Virginia (UVA) and a separate colony established. At both institutions, the mice were housed and treated in accordance with the NIH guide to Humane Use of Animals in Research. All surgical procedures were approved by the Animal Care and Use Committees at UVA and NIEHS. Genotyping was performed as previously described.34 Full characterization of the phenotypic, biologic and biochemical properties of these animals have been published.34 Results obtained from the collaborative studies between the 2 institutions and using identical protocols were pooled for statistical analysis presented in Figure 3a.
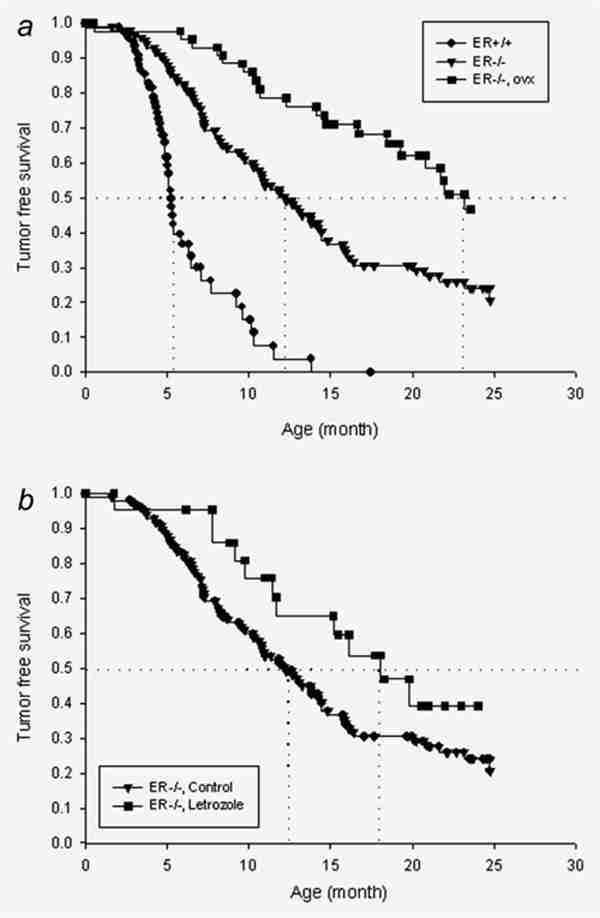
(a) Kaplan–Meier curves of tumor formation in noncastrate ER+/+/Wnt-1 (n = 79) and ERKO/Wnt-1 animals (n = 120) and the effect of oophorectomy performed before 16 days of age on tumor formation in ERKO/Wnt-1 animals (n = 48). The vertical dotted lines represent the 50% incidence time point as described in the text. The curves were drawn with pooled data from NIEHS and the University of Virginia. The differences among 3 groups were statistically significant (p < 0.001). (b) Kaplan–Meier curves of tumor-free survival of intact ERKO/Wnt-1 animals treated with (n = 24) or without (n = 120) letrozole (20 µg/mouse/day). The difference between these curves was marginally significant (p = 0.055).
“E2 clamp” method and drug administration
Silastic tubes of 0.19 cm internal diameter were filled with E2/cholesterol mixtures at various ratios. The lengths of the filled part of Silastic tubes were 2.5, 5 or 7.5 mm, respectively. Our prior studies validated the ability to “clamp” plasma E2 at levels ranging from 20 to 800 pg/ml over a 2-month period37 and demonstrated linear dose responses in uterine weight in castrate mice.38 In our study, plasma E2 was “clamped” at levels representing postmenopausal (5 and 10 pg/ml), early follicular phase (80 pg/ml) and midluteal phase (240 pg/ml) levels in women. The implants used contained the following E2/cholesterol ratios and lengths: 1:39/2.5 mm (5 pg/ml), 1:19/2.5 mm (10 pg/ml), 1:3/2.5 mm (80 pg/ml) and 1:3/7.5 mm (240 pg/ml). Implants were inserted under the skin in the backs of the mice and changed every 2 months. Fulvestrant dissolved in sesame oil was administered by subcutaneous injection once per week at a dosage of 5 mg/mouse. Letrozole was suspended in 0.3% carboxymethyl cellulose solution in saline and administered by subcutaneous injection once a day at a dosage of 20 µg/mouse, 5 days a week. The complete blocking effects of fulvestrant were demonstrated by bioassay of uterine weight as described later.
Bioassay of ER-dependent actions of E2
Measurements of uterine wet weight were used as a bioassay for ER-dependent actions of E2. Ovariectomy was carried out at 15 days of age. Treatment with E2 via silastic implants at a dose of 240 pg/ml alone or in combination with fulvestrant at 5 mg/week subcutaneously started immediately after ovariectomy and continued for 2 months. At the end of treatment, animals were anesthetized and uteri were dissected and weighed after blotting of fluid. In experiments involving assessment of tumor formation, uteri were collected and weighed at the animal sacrifice when tumors were detected or at the end of the experiment without tumor formation. No increase in uterine weight occurred as a function of age in postpubertal animals (Supporting Information Figure 1).
Preparation of whole mounts
The whole mounts from excised mammary glands were fixed and stained as previously described.39 The inguinal mammary glands were excised, placed on glass slides and immerged in Carnoy’s fixative for 2–4 hr at room temperature. The glands were washed with 70% ethanol for 15 min, gradually hydrated and then stained overnight in carmine alum solution (1 g carmine natural red, 2.5 g aluminum potassium sulfate in 500 ml distilled water and a crystal of thymol). The glands were dehydrated progressively in 70, 95 and 100% ethanol for 15 min during each step. The mammary fat pads were cleared in xylene. The mammary whole mounts were photographed using Olympus SZX12 microscope.
Endogenous and exogenous gene expression assays
The ERE-TATA-luciferase reporter system was previously described in detail.40 Progesterone receptors A and B were detected on Western blots using a monoclonal antibody against the progesterone receptor (Cell Signaling Technology, Beverly, MA).40
Statistical methods
The Kaplan–Meier analyses were used to compare the tumor-free survival time between different treatment groups of mice in the study. The rate of tumor development was compared among the various treatment groups to determine whether they are statistically significantly different. The Student’s t-test was used to compare mean uterine weights between 2 groups and a significance level of 0.05 was considered to be statistically significant.
Results
E2 effects on mammary proliferation in ERKO mice
To demonstrate that E2 did not induce proliferative effects on breast tissue in the ERKO/Wnt-1 model, we initially conducted systematic examination of mammary whole mounts. Our prior studies had demonstrated that knockout of ERα36 allowed development of only rudimentary mammary ductal structures (Supporting Information Figure 2B) but that introduction of the Wnt-1 gene into ERKO animals caused proliferation of the existing mammary rudiment (Supporting Information Figures 2D and 2F). Our current studies demonstrated that E2 did not influence proliferation of breast tissue in the absence of ERα. As we showed before, the mammary gland was fully developed in mice bearing wild-type ERα. Wnt-1 expression caused mammary gland hyperplasia (Fig. 2, bottom panel: intact). Removal of ovaries reduced the size of lobules, which could be reversed by administration of estradiol. The morphology of glands from mice receiving E2 plus fulvestrant was the same as that in ovariectomized mice (Fig. 2, bottom panel: ovx + E2 + ICI). The results implicated an important role of E2 in stimulation of lobular proliferation even in the presence of Wnt-1. In striking contrast, no substantial change in mammary gland morphology occurred in ERKO/Wnt-1 mice when the ovaries were removed (Fig. 1, top panel: ovx). More importantly, supplementation with 240 pg/ml E2 for at least 2 months did not stimulate lobule proliferation (Fig. 1, top panel: ovx + E2). Administration of fulvestrant (Fig. 1, top panel: ovx + E2 + ICI) did not alter mammary gland morphology in ERKO/Wnt-1 mice. To further examine proliferation, proliferating cell nuclear antigen (PCNA) in mammary glands of mice expressing wild-type ERα or with ERα knocked out was analyzed by Western blot. The average level of PCNA in ERKO mammary gland was less than 20% of that in ER+ gland (data not shown) even though the circulating estradiol in ERKO mice is 10-fold higher than in ER+ mice.41 These data provided evidence that knockout of ERα prevented proliferative effects of E2 on mammary gland in this model.
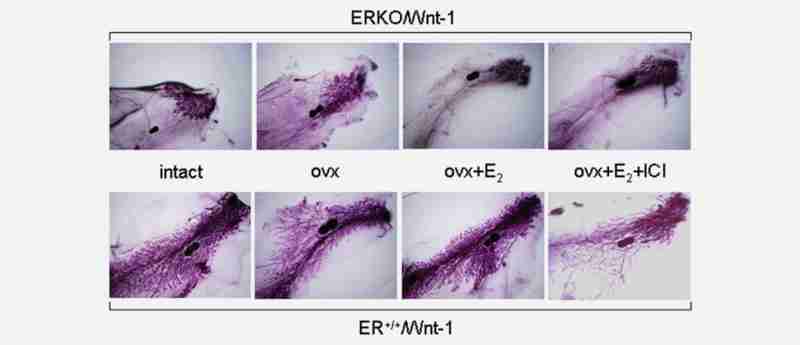
Whole mounts of the mammary gland in ERα knockout (ERKO) animals cotransfected with the Wnt-1 gene (ERKO/Wnt-1, top panels) and in ERα positive wild-type animals bearing the Wnt-1 gene (ER+/+/Wnt-1, bottom panels). The entire mammary fat pad is shown with the central lymph node (dense blue) shown on each whole mount for orientation. Three to six animals from each group were examined and morphology of the mammary gland within each group was highly consistent. The groups include: intact: animals in which the ovaries have not been removed surgically; ovx: animals in which the ovaries have been removed surgically before day 16 of life; ovx + E2: animals whose ovaries have been surgically removed but received E2 with silastic implants designed to produce plasma levels of 240 pg/ml; and ovx + E2 + ICI: animals having undergone surgical removal of the ovaries and receiving E2 implants (240 pg/ml) plus fulvestrant (ICI, 5 mg/mouse/week).
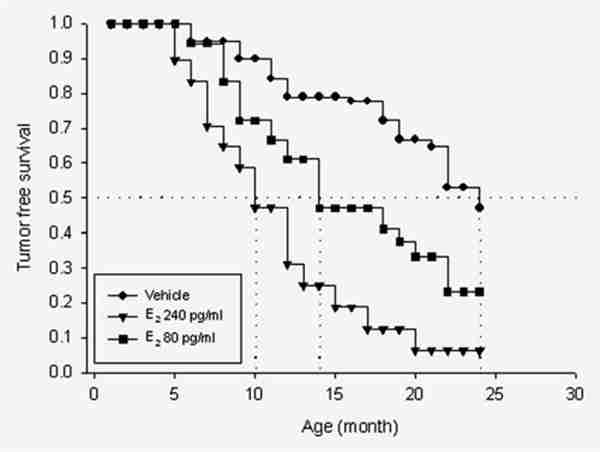
Kaplan–Meier curves comparing the effect of estradiol on tumor formation in oophorectomized, ERKO/Wnt-1 animals treated either with cholesterol (vehicle, n = 20) or with silastic implants producing plasma midluteal phase levels of estradiol (240 pg/ml, n = 20) or early follicular phase levels (80 pg/ml, n = 19). The differences between the vehicle-treated animals and those with plasma E2 levels clamped at 240 pg/ml were statistically significant (p = 0.0002). The animals with levels clamped at 80 pg/ml exhibited tumor development curves intermediate between those in the vehicle treated and those receiving 240 pg/ml.
ERα-independent effects of E2 on tumor development
We administered E2 or vehicle over a 24-month period to castrate ERKO/Wnt-1 animals to examine the effects of E2 on tumor formation in animals lacking ERα. With the “E2 clamp” methodology,42 we maintained plasma E2 at levels reflecting postmenopausal (5 and 10 pg/ml), early-follicular (80 pg/ml) and midluteal (240 pg/ml) phase concentrations in women. Two endpoints were utilized to assess effects of E2 administration: (1) the percentage of animals developing tumors and (2) the time in months at which 50% of the animals developed tumors (50% incidence time). Regarding the first end point, 50% of castrate animals treated with vehicle developed tumors over 2 years. In marked contrast, 80% of animals receiving follicular phase levels of E2 developed tumors and nearly 100% in those with luteal phase levels. Tumor incidence in animals receiving postmenopausal levels of E2 did not differ from those given vehicle (data not shown). With respect to the second end point, the 50% incidence time in the vehicle and postmenopausal E2-treated animals was 23 months. A striking difference was observed in animals with E2 “clamped” at luteal phase levels of 240 pg/ml, whose 50% incidence time point was 10 months (p = 0.0002). Those with follicular phase levels had an intermediate 50% incidence time point of 14 months (Fig. 2).
Effect of castration on tumor development in ERKO/Wnt-1 animals
As exogenous E2 increased tumor incidence and reduced latency, we reasoned that castration, by lowering endogenous E2, should also reduce tumor incidence from levels observed in intact ERKO/Wnt-1 animals. For these experiments, we compared intact and castrate ERKO/Wnt-1 animals. Castration delayed tumor onset (50% incidence time) from 12 to 23 months and reduced tumor incidence from 80% to 50% (p < 0.001).34 Our working hypothesis is that both ERα dependent and ERα independent effects of estradiol are involved in carcinogenesis. This experiment also allowed verification of the expected ERα dependent effect on the process of carcinogenesis. Tumors developed sooner (50% tumor incidence time was 6 months) in the ER+/+/Wnt-1 animals than in those lacking ERα (Fig. 3a).
Since castration reduced tumor incidence in the intact ERKO animals, we reasoned that pharmacologic suppression of estrogen production should also exert similar effects. We had previously developed a regimen sufficient to block ovarian estrogen production to castrate levels in rodents with high-dose letrozole (AI) administration.43 Letrozole, given at a dosage of 20 lg/day for 5 days a week, increased 50% tumor incidence time from 12 to 18 months (p = 0.055) (Fig. 3b). The effect of letrozole was dose-dependent, since no difference in 50% incidence time point or overall incidence was observed in animals receiving a lower dose (10 µg/day) of AI versus vehicle (data not shown).
Complete elimination of effects of truncated ERα and ERβ
A factor confounding our experimental design is that the “Korach” ERKO animals retain 5–20% of the ERα activity present in their ER+ counterparts. To eliminate these effects, we blocked ERα (and ERβ) function with the “pure antiestrogen” fulvestrant and examined the effect of E2 under these conditions. Fulvestrant or vehicle was administered to castrate ERKO/Wnt-1 animals with E2 “clamped” at 240 pg/ml. As evidence of an ER independent effect, E2 induced tumor formation similarly in the E2/fulvestrant treated as in the E2/vehicle-treated animals (Fig. 4a). To provide further support that residual ERα did not explain our results, we administered 17α-OH-E2, an estrogen analogue lacking ERα-mediated activity but capable of metabolism to potentially genotoxic metabolites.44 Our data demonstrated that 17α-OH-E2 induced tumors in the castrate ERKO/Wnt-1 animals at a rate similar to that in animals with E2 maintained at the same plasma level (i.e., 240 pg/ml) (Fig. 4b).
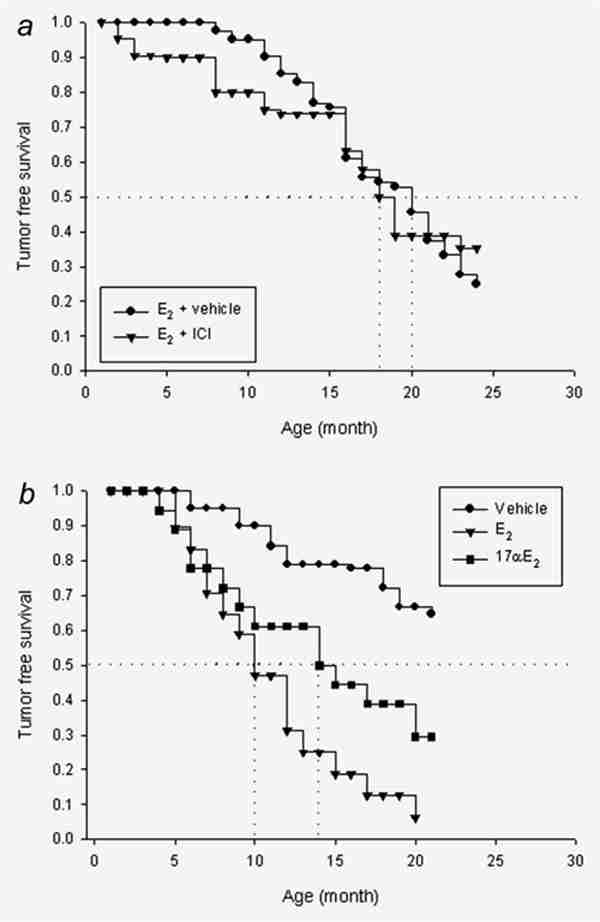
(a) Kaplan–Meier curves comparing the effect of 240 pg/ml of E2 with or without fulvestrant (ICI) in castrate ERKO/Wnt-1 animals (n = 21). These curves were not statistically significantly different (p = 0.56). (b) Kaplan–Meier curves showing the effect of 17α-OH-E2 on tumor formation in ERKO/Wnt-1 animals receiving 240 pg/ml 17α-OH-E2 (n = 18). The difference between 17α-OH-E2 and 17β-OH-E2 was not statistically significant (p = 0.34). Data on the 240 pg/ml E2 and vehicle doses are reproduced from Figure 2 and are statistically significant (p = 0.0002).
Bioassay of “clamped” E2 on uterine weight
Our various strategies to examine the ER-independent effects of E2 critically depended on complete blockade of any residual ER activity resulting from a truncated ERα or from low levels of ERβ. Measurement of uterine weight provided a robust bioassay of E2 to determine whether complete blockade was achieved. We measured uterine weight after at least 2 months of E2 exposure under each experimental condition (Fig. 5). In the ERKO castrate animals, E2 stimulated uterine weight to 18% of that observed in ER+/+/Wnt-1 animals, an effect resulting from a biologic effect of the truncated 56KD receptor (Fig. 5). Fulvestrant completely blocked the residual ER responsiveness in the ERKO/Wnt-1 animals. Uterine weight fell to 7 ± 1 mg in the animals receiving 240 pg/ml E2 plus fulvestrant, a uterine weight similar to that observed in castrate animals (Fig. 5). In aggregate, these data demonstrated that fulvestrant was capable of completely abrogating the effects of residual ER activity in ERKO animals.
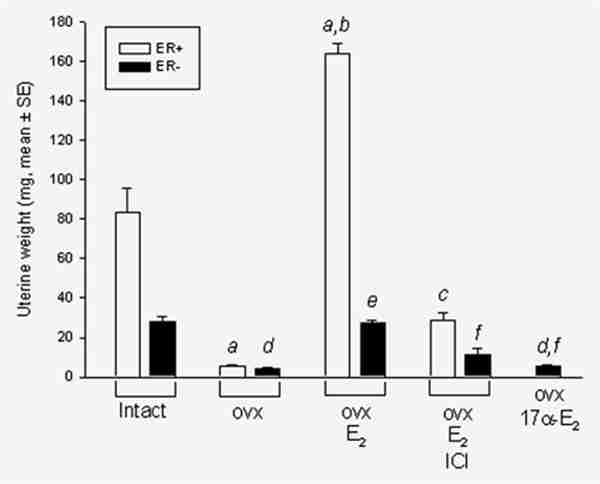
Uterine wet weights in ER+/Wnt-1 (ER+, white bars) and ERKO/Wnt-1 (ER−, black bars) animals. Shown are mean (±SE) weights of the uterus under various conditions. Data from ER-animals (ovx and E2 groups) were pooled from different experiments (n = 48). 17α-E2/ovx: castrate animals receiving 17α-OH-E2 to produce plasma levels of 240 pg/ml (n = 17). Statistical analysis: ER+ groups: (a) compared to intact (n = 6), (b) compared to ovx (n = 3), (c) compared to ovx + E2 (n = 5); ERKO groups: (d) compared to intact (n = 39), (e) compared to ovx (n = 13), (f) compared to ovx + E2 (n = 48) with all p-values less than 0.001.
We also wished to confirm by bioassay that 17α-OH-E2 did not exert ER-mediated effects as further evidence that its activity on tumor formation was ER-independent. At a level of 240 pg/ml, this compound caused no stimulation in uterine weight (4 ± 0.5 mg) indicating its lack of uterotropic activity (Fig. 5). Specifically, we observed no stimulation of uterine weight in ER+/+ mice by 4-day injections of 17α-OH-E2, whereas the same doses of 17β-E2 caused a 3-fold increase in uterine weight (Supporting Information Figure 3). As further proof of the minimal ER-mediated effects of 17α-OH-E2, we tested its ability to stimulate transcription of endogenous and exogenous estrogen responsive genes and MCF-7 cell growth in vitro. The growth of MCF-7 cells in response to 17β-E2 and 17α-OH-E2 was evaluated by cell counting after 5-day exposure to these steroids. The potency of 17α-OH-E2 on transcription of an ERE-luciferase construct (exogenous reporter gene), on progesterone receptor synthesis (endogenous genes) and on cell growth was 1% or less than that of E2 (Supporting Information Figures 4A–C).
Discussion
Basic, epidemiologic and clinical studies provide strong evidence of a role for estrogen in the genesis of breast cancer but the precise mechanistic actions on tumor formation are incompletely understood.9,11,13,24,45,46 While compelling biologic and clinical evidence support a key role for ERα mediated effects, receptor independent pathways involving estrogen metabolites may also contribute to breast cancer initiation.25 Our study utilized a “reductionist” approach to provide in vivo support for the principle that estrogens could influence mammary tumor formation in the absence of functioning estrogen receptors. Use of an ERKO/Wnt-1, double transgenic, castrate mouse model allowed specific assessment of the role of exogenous E2 on tumor formation while “reducing” the confounding effects of prolactin, progesterone and other ovarian factors. Inhibition of endogenous E2 by oophorectomy or an AI in ERKO mice provided another means of assessment. Using these various experimental approaches, we demonstrated statistically significant, dose-related effects of E2 on breast tumor onset and incidence in the absence of a functioning ERα.
While there was no functional ERα in our model, interpretation of our results could be confounded by the effects of residual ERα, ERβ and GPR30, a membrane associated protein that mediates nongenomic effects of E2. The “Korach” ERKO mice express an mRNA species that yields a 56 Kd truncated ERα message41 whose translated protein retains its DNA and ligand binding domains. The truncated ERα has less than 10% of the biological activity of the native ERα. To minimize the possible confounding effects, we administered the “pure” antiestrogen fulvestrant to block the activities of residual ERα. Complete blockade of ERα activity by fulvestrant was confirmed by using uterine weight as a bioassay. Another approach to demonstrate a non-ER mediated effect was the use of 17α-OH-E2, a metabolizable but ER impeded estrogen. 17α-OH-E2 significantly influenced tumor onset and incidence in ERKO animals but lacked uterotrophic activity.
Several studies suggest that ERβ exerts minimal effects on rodent mammary glands.47 Expression of ERβ mRNA could only be detected by PCR methodology in mammary tissue of ERKO mice.48 Nevertheless, if ERβ were present and important for tumor formation, fulvestrant would block the activity of this receptor. Accordingly, our data provide strong evidence that ERβ did not explain our results. The G-protein coupled receptor GPR30 was recently reported to mediate estrogen promoted proliferative signaling in an ER-negative breast cancer cell line and human endometrial cells. However, 2 recent studies using GPR30−/− mice indicated that GPR30 does not mediate estrogenic responses in the uterus and the mammary gland.49,50 In addition, in vitro studies showed that fulvestrant did not inhibit but paradoxically activated GPR30.51 The ER impeded analogue, 17α-OH-E2 could not activate GPR30.51 Our results with fulvestrant and 17α-OH-E2 provided indirect evidence that GPR30 did not play a role in mammary tumor initiation in our model. Taken together, these multiple experimental approaches provided strong evidence in support of the principle that E2 can influence breast tumor development independently of ER functionality.
The ER independent effects of E2 on tumor formation likely occur via estrogen metabolites. As reviewed by Yager and Davidson, estrogens are hydroxylated at the 2-, 4- and 16α-positions.24 The 2 and 4 catechol estrogen metabolites are involved in redox cycling with generation of oxygen free radicals, which can cause DNA damage. An additional genotoxic mechanism involves further oxidation to the 2,3- and 3,4-estradiol-quinones, which form covalent adenine and guanine DNA adducts. The adenine and guanine adducts of the 4-hydroxylated products are unstable and result in cleavage from DNA with formation of depurinated sites.25 Mutation at these DNA sites can occur through error prone DNA repair. Our prior measurements in rodent and human mammary tissue demonstrated that both benign and malignant breast tissue are able to metabolize E2 to E2-3,4-quinones that react with DNA to form depurinating N3Adenine and N7Guanine adducts.52
Estrogen hydroxylation at the 2 and 16 positions appears to be less important than 4-hydroxylation for genesis of tumors. Adducts arising from 2-hydroxylated-estrogen metabolites depurinate minimally and are found at very low levels in women and men with cancer.28,29,53,54 Animal studies46 demonstrate that 4-OH-E2 but not 2-OH-E2 causes kidney and uterine cancer. Although a series of prior studies suggested the importance of 16α hydroxylation in cancer formation,55 recent reviews question this conclusion based on the lack of specificity of earlier assays used to measure these compounds.24,46
Comprehensive data from several published in vitro studies provide additional experimental support for the “genotoxic E2 metabolism hypothesis.”24,25,31,32,46,56 Estradiol caused DNA point mutations in ER negative V-79 and Big Blue rat cell culture mutation assays.30,31 Benign, ER negative MCF-10 mammary epithelial cells, when exposed to physiologic concentrations of E2, underwent malignant transformation and form tumors in immunodeficient mice.25,57 At physiologic concentrations, E2 caused loss of heterozygosity in MCF-10 cells at “hot spots,” which are commonly observed in human mammary tumors.25,32 Hormonally active but non-metabolizable estrogens exhibited a reduced ability to cause cancer and inhibitors of estrogen metabolism reduce the incidence of estrogen induced kidney tumors in the Syrian hamster.46
Clinical and epidemiologic data also support the possible biologic relevance of ER independent effects of E2 on tumor formation. In patients carrying the BRCA1 gene mutations, bilateral oophorectomy reduces the risk of breast cancer by 53%.58 Since only 10–24% of breast tumors in BRCA1 mutation carriers are ER+,59,60 it has been suggested that the protective effects of oophorectomy might occur independently of ERα.61,62 Women at high risk of breast cancer excrete larger amounts of depurinated adenine and guanine-estradiol conjugates than women at low risk of breast cancer.28,29 In pre-menopausal patients with mutations of multiple estrogen metabolizing genes, the risk of breast cancer has been reported to be 4-fold higher than in controls lacking these mutations.26 However, it should be noted that studies of patients with mutations of only 1 of these genes have reported conflicting results regarding breast cancer risk.27
We acknowledge that several confounding factors could have influenced the interpretation and validity of our results. The Wnt-1 gene is expressed in mammary tissue because of the LTR of the mouse mammary tumor virus. Increased tumor incidence in E2-treated animals might be an indirect result of altered Wnt-1 expression. However, our prior studies carefully examined the expression of Wnt-1 in the ERKO and wild-type animals and showed no alteration of expression.41 We also examined Wnt-1 expression by Western analysis of mammary gland and tumor samples from ER+/+/Wnt-1 and ERKO/Wnt-1 mice in our study and found no change in Wnt-1 levels at different period of experiments or with E2 treatment (Supporting Information Figure 5). These results render unlikely an alteration of Wnt-1 expression as an explanation of our results. Epigenetic breast imprinting in utero caused by an absence of ERα functionality could have resulted in increased susceptibility to cancer in our animal model. However, breast cancer is influenced by factors occurring in utero in women,63,64 and thus our model could possibly reflect such effects.
Several experiments pooled animals from our 2 institutions (UVA and NIEHS) to enhance the sizes of experimental groups. Justification for pooling included the use of identical protocols and the comparability of mean tumor onsets among groups (Supporting Information Table 1). Our studies were conducted over a period of several years (a problem caused by the slow onset of tumors in this model) and involved several independent experiments. This likely explains the variability in time of onset of tumors among the various experiments. This variability clearly confounded the E2 alone versus E2 plus fulvestrant experiment. Nonetheless, the data clearly ruled out residual effects of ERα on tumor formation as assessed by the direct concomitant comparison of E2 alone versus E2 plus fulvestrant even though the 50% tumor incidence time in both groups was delayed from other experiments (i.e., 18–20 months). It should be noted that tumor incidence in the vehicle alone groups in other experiments ranged from 23 to 24 months. While this variability was clearly a limitation of the study, 2 other observations also argue against an ERα effect. Exogenous E2 did not alter breast morphology in ERKO animals (Fig. 1). And 17α-estra-diol enhanced tumor development but did not stimulate uterine weight.
In summary, our data provided strong evidence supporting the principle that breast cancer development can be influenced by E2 via ER independent mechanisms. A necessary component of the proof was to fulfill Koch’s third postulate which requires the disease to be produced by administration of the putative etiologic factor, in this case, E2.65 While other actions of tamoxifen such as epigenetic and genotoxic activities might be involved,66–68 our study provides important mechanistic evidence in support of the use of AIs in preference to the antiestrogens for prevention of breast cancer. As shown in the cartoon in Figure 6, antiestrogens primarily block receptor mediated pathways whereas the AIs block both receptor mediated and receptor independent effects of E2. Two current clinical trials are examining the AIs for prevention of breast cancer.69,70 Finally, a speculative consideration for the future is that blockade of estradiol metabolism with CYP1B1 and 1A1 inhibitors might be a means to reduce breast cancer incidence without blocking formation of E2 itself.
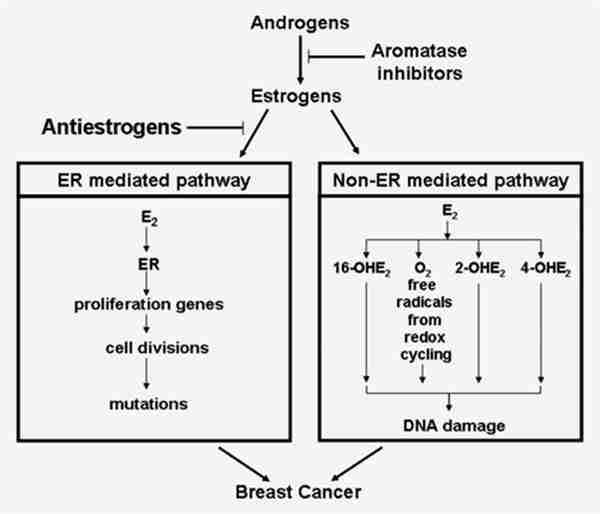
Diagrammatic representation of effects of an antiestrogen and an aromatase inhibitor on prevention of breast cancer. This model postulates that antiestrogens only block ERα mediated effects on breast cancer whereas the aromatase inhibitors, by inhibiting estrogen synthesis, abrogate both ERα mediated as well as the genotoxic effects of estrogen.
Footnotes
Additional Supporting Information may be found in the online version of this article