- Journal List
- Informa Healthcare Open Access
- PMC4266039
Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives
Tessa Moses
1Department of Metabolic Biology, John Innes Centre, Colney Lane, Norwich, UK
Kalliope K. Papadopoulou
2Department of Biochemistry and Biotechnology, University of Thessaly, Larisa, Greece
Anne Osbourn
1Department of Metabolic Biology, John Innes Centre, Colney Lane, Norwich, UK
 Corresponding author.
Corresponding author.Abstract
Saponins are widely distributed plant natural products with vast structural and functional diversity. They are typically composed of a hydrophobic aglycone, which is extensively decorated with functional groups prior to the addition of hydrophilic sugar moieties, to result in surface-active amphipathic compounds. The saponins are broadly classified as triterpenoids, steroids or steroidal glycoalkaloids, based on the aglycone structure from which they are derived. The saponins and their biosynthetic intermediates display a variety of biological activities of interest to the pharmaceutical, cosmetic and food sectors. Although their relevance in industrial applications has long been recognized, their role in plants is underexplored. Recent research on modulating native pathway flux in saponin biosynthesis has demonstrated the roles of saponins and their biosynthetic intermediates in plant growth and development. Here, we review the literature on the effects of these molecules on plant physiology, which collectively implicate them in plant primary processes. The industrial uses and potential of saponins are discussed with respect to structure and activity, highlighting the undoubted value of these molecules as therapeutics.
Introduction
Saponins are naturally occurring structurally and functionally diverse phytochemicals that are widely distributed in plants. They are a complex and chemically varied group of compounds consisting of triterpenoid or steroidal aglycones linked to oligosaccharide moieties. The combination of a hydrophobic aglycone backbone and hydrophilic sugar molecules makes the saponins highly amphipathic and confers foaming and emulsifying properties. These surface-active molecules have important roles in plant ecology and are also exploited for a wide range of commercial applications in the food, cosmetic and pharmaceutical sectors. Saponins in Quillaja saponaria extracts are used as foaming agents in carbonated beverages and cosmetics, as emulsifiers in preparations containing lipophilic colors or flavors, as preservatives, and for removal of dietary cholesterol (Güçlü-Ustündağ & Mazza, 2007; San Martín & Briones, 1999). Likewise, licorice saponin extracts are used as flavor modifiers in baked foods, chewing gum, beverages, candies, herbs, seasonings and dietary supplements (Güçlü-Ustündağ & Mazza, 2007). Saponins are also the major constituents of traditional folk medicines such as the ginsenosides produced by Panax species (Qi et al., 2011). A number of therapeutic properties have been ascribed to saponins (Francis et al., 2002). These molecules are potent membrane permeabilizing agents. They are also immunostimulatory, hypocholesterolemic, anti-carcinogenic, anti-inflammatory, anti-microbial, anti-protozoan, molluscicidal and have anti-oxidant properties. Furthermore, they can impair gut protein digestion, and uptake of vitamins and minerals. In general, the saponins and their biosynthetic intermediates display an array of properties that can have positive or negative effects in different animal hosts (Francis et al., 2002; Sparg et al., 2004).
Although the saponins are one of the largest classes of plant natural products, their biological functions are not completely understood. They are generally considered to have important roles in defense of plants against pathogens, pests and herbivores due to their antimicrobial, antifungal, antiparasitic, insecticidal and anti-feedant properties (Augustin et al., 2011; Morrissey & Osbourn, 1999; Osbourn et al., 2011; Sparg et al., 2004). Many plants synthesize and accumulate saponins during normal growth and development. The distribution of these natural products varies greatly among plant species, individual plants, organs and tissues, during development and maturation, and shows seasonal fluctuations. Some studies have suggested that variations in saponin distribution, composition and amounts in plants may be a reflection of varying needs for plant protection. In Phytolacca dodecandra and Dioscorea pseudojaponica, maximal saponin accumulation has been noted during fruit and tuber development, and has been suggested to prevent fruit loss, ensure seed maturation and protect reproductive organs (Lin et al., 2009; Ndamba et al., 1994). However, in several plant species, the production of saponins is induced in response to biotic stress including herbivory and pathogen attack. Abiotic stress factors such as humidity, nutrient starvation, light and temperature can influence both the quality and quantity of saponin content (De Costa et al., 2013; Szakiel et al., 2011). Increase in saponin levels in response to stress is often mediated by the transcriptional activation of biosynthetic genes through a complex signaling cascade involving the jasmonate and salicylate hormones. Hence, the biosynthesis of these molecules can be induced using elicitors and this feature has been exploited in several plant species to improve saponin yields (Shabani et al., 2009; Yendo et al., 2010).
Here, we provide a brief introduction to the vast diversity of saponin structures that are synthesized and accumulated by different plant species. Emphasis is given to differences in the composition and quantity of these natural products over different phases of plant growth, development and maturation. We provide a constructive framework to suggest that these compounds, which are traditionally regarded as secondary metabolites, have important roles in plants at various stages of development, including seed germination, vegetative growth and differentiation, fruiting and nodulation. A growing number of studies have reported effects on plant growth and development due to perturbation of saponin biosynthesis, suggesting roles for these molecules and their intermediates in primary plant processes. With these data, we highlight that maintaining a delicate balance of saponin biosynthesis is essential for overall plant integrity and well-being. The industrial applications of saponins, their intermediates and derivatives are also reviewed, together with structure–activity relationship data, to provide the reader with a perspective on the importance of this class of metabolites for pharmaceutical applications.
Biosynthesis of saponins
Most known saponins are found in angiosperms (Magnoliophyta), though some marine invertebrates such as sea cucumbers (Holothuroidea) and starfish (Asteroidea) also produce these molecules (Bordbar et al., 2011; Liu et al., 2008; Osbourn et al., 2011; Williams & Gong, 2007). While the synthesis of saponins is widespread in plants, the majority of the producing plant species is dicotyledonous and accumulates mainly triterpenoid-type saponins. The monocotyledonous angiosperms on the other hand mostly, but not exclusively, synthesize steroidal-type saponins. This broad classification of saponin types is based on the nature of the aglycone backbone from which the saponin molecule is derived. Both triterpenoid and steroidal aglycone backbones are derived from the 30-carbon linear precursor 2,3-oxidosqualene. During the synthesis of the committed precursor, the steroidal aglycone loses three methyl groups to result in a 27-carbon backbone, whereas the triterpenoid aglycone retains all 30 carbons in its backbone. The steroidal glycoalkaloids, which are also sometimes referred to as saponins, share their biosynthetic origin with the steroidal saponins and contain a characteristic nitrogen atom incorporated into the aglycone backbone (Augustin et al., 2011; Friedman, 2006; Ginzberg et al., 2009; Itkin et al., 2013).
The triterpenoid and steroidal aglycone backbones are isoprenoids that are synthesized from isopentenyl pyrophosphate (IPP) units generated by the mevalonate (MVA) pathway (Figure 1). The multistep MVA/3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) pathway catalyzes the conversion of acetyl-CoA to the five-carbon terpene precursor IPP, which is then isomerized to its allylic isomer dimethylallyl pyrophosphate (DMAPP) by the enzyme isopentenyl diphosphate isomerase (IDI). The subsequent condensation of two IPP units with one DMAPP unit results in the formation of 15-carbon farnesyl pyrophosphate (FPP), the immediate prenylated precursor of the saponins. Finally, the condensation of two FPP units by squalene synthase (SQS) generates the linear 30-carbon precursor squalene, which is further epoxidized to 2,3-oxidosqualene by the enzyme squalene epoxidase (SQE). The 2,3-oxidosqualene is typically cyclized by a variety of oxidosqualene cyclases (OSCs) to polycyclic structures, which in higher plants marks the branch point between primary and specialized triterpene metabolism. The tetracyclic, primary triterpene precursor cycloartenol is generated by the cyclization of 2,3-oxidosqualene by cycloartenol synthase (CAS). In angiosperms, a mixture of phytosterols are generated from cycloartenol, including the 27-carbon cholesterol, the 28-carbon campesterol and the 29-carbon sitosterol. The steroidal saponins are synthesized by a series of oxygenations and glycosylations of the cholesterol backbone to spirostanol or furostanol derivatives with a fused O-heterocycle in their core aglycone structure (Thakur et al., 2011). The steroidal glycoalkaloids also utilize cholesterol as the committed precursor, but incorporate an amine group through side chain modifications to generate aglycones such as solasodine, solanidine, demissidine and tomatidine (Ginzberg et al., 2009; Itkin et al., 2013). The aglycones are then tailored with oxidoreductases before being glycosylated with multiple sugar moieties (Friedman, 2006).
Overview of structural diversity in saponin aglycones. The saponin biosynthesis pathway begins with acetyl-CoA, which via the mevalonate pathway is converted to the linear 30-carbon precursor squalene (yellow). Squalene is oxidized to 2,3-oxidosqualene, which is cyclized to the cycloartane aglycone, the dedicated precursor for sterol biosynthesis in plants (green). Cholesterol is the dedicated precursor for the synthesis of steroidal saponins (violet) and steroidal glycoalkaloids (blue). The triterpenoid saponins (orange) are synthesized from 2,3-oxidosqualene that is cyclized to specialized triterpene aglycones. Enzyme and pathway names are italicized. Dashed arrows imply multiple steps in the pathway.
All the remaining cyclization products of 2,3-oxidosqualene are committed precursors for the synthesis of specialized triterpenes. This cyclization reaction, involving multiple carbocation rearrangement steps, generates the first level of structural diversity inherent to the triterpenoid saponin aglycones, as the single substrate 2,3-oxidosqualene can be cyclized to an array of triterpene scaffolds. As such, nine main classes of triterpene backbones have been documented to be synthesized by plants (shown in orange in Figure 1; Vincken et al., 2007). The OSCs catalyzing these reactions are either specific or multifunctional in nature, and cyclize 2,3-oxidosqualene to a single product or to multiple products derived from a particular cyclization pathway in a single reaction. The triterpene aglycones are then modified, mainly oxidized, by a series of cytochrome P450-dependent monooxygenases (P450s). These modifications add a second level of complexity and expand the structural diversity of the backbone. Consecutive oxidations at different positions of the triterpene backbone increase the polarity of the scaffold and introduce reactive functional groups which are subsequently modified by an array of transferases, including UDP-dependent glycosyltransferases (UGTs), acyltransferases, malonyltransferases and methyltransferases. A comprehensive overview of the biosynthesis of triterpenoid saponins can be found in Thimmappa et al. (2014).
The genes involved in the biosynthesis of all three types of saponins (triterpenoid, steroid and steroidal glycoalkaloid) can therefore be grouped into four main categories based on their characteristic reactions, and include the OSCs, P450s, UGTs and other tailoring enzymes (mostly encoding transferases). Recently, numerous examples of gene clusters for the biosynthesis of different classes of specialized metabolites have been discovered in a variety of plant species, including clusters for saponin biosynthesis (Boycheva et al., 2014; Nützmann & Osbourn, 2014). Biosynthetic gene clusters have been identified for triterpenoid saponins and steroidal glycoalkaloids. The first triterpene gene cluster to be identified was the avenacin cluster from Avena strigosa (oat), which contains five biosynthetic genes encoding for an OSC, a P450, a UGT and two other tailoring enzymes (a methyltransferase and a serine carboxypeptidase-like acyltransferase) (Haralampidis et al., 2001a; Mugford et al., 2013; Papadopoulou et al., 1999; Qi et al., 2004, 2006). Other closely linked genes that form part of this cluster have been defined by genetics but not cloned (Mylona et al., 2008; Papadopoulou et al., 1999; Qi et al., 2004). In Arabidopsis thaliana, two triterpenoid gene clusters have been identified for the biosynthesis of thalianol- and marneral-derived triterpenes, containing four and three genes, respectively. The thalianol cluster is comprised of genes encoding an OSC, two P450s and an acyltransferase (Field & Osbourn, 2008). Likewise, the marneral cluster encodes an OSC and two P450s (Field et al., 2011). A triterpenoid gene cluster encoding an OSC and two P450s has also recently been identified in Lotus japonicus (Krokida et al., 2013). Gene clusters for the synthesis of the steroidal glycoalkaloids α-tomatine and α-chaconine/α-solanine have been discovered in Solanum lycopersicum (tomato) and Solanum tuberosum (potato), respectively (Itkin et al., 2013). The tomato gene cluster consists of eight characterized biosynthetic genes encoding for two P450s, four UGTs and two other tailoring enzymes (a dioxygenase and a transaminase). Similarly, the potato cluster contains six characterized genes encoding for two P450s, two UGTs, a dioxygenase and a transaminase. Although once thought to be an exception, the phenomenon of physical clustering of biosynthetic pathway genes for different types of secondary metabolites in plants is becoming common (Nützmann & Osbourn, 2014). The physical clustering of genes in the genome together with co-ordinate expression of the clustered genes is proving to be a useful tool to identify biosynthetic genes involved in new pathways for the synthesis of specialized metabolites (Castillo et al., 2013; Field & Osbourn, 2008; Field et al., 2011; Nützmann & Osbourn, 2014).
Enzymes encoded by multigene families for OSCs, P450s, UGTs and other specialized transferases are the key actors in the biosynthesis of plant triterpenoid, steroidal and steroidal glycoalkaloid saponins. Although the number of characterized enzymes belonging to these gene families has increased over the last decade, there is still a wide gap in our understanding of saponin biosynthesis, in how the synthesis of these molecules in different plant tissues and organs is regulated during development and in response to environmental stimuli, and in how these molecules are transported. Unraveling these molecular mechanisms will provide a better understanding of the role of saponins in plant processes, and will guide their exploitation in industrial applications.
Occurrence and distribution in plants
Saponins are synthesized and accumulated by many different plant families (Dinda et al., 2010; Sparg et al., 2004; Vincken et al., 2007). They occur in both wild and domesticated plants, with the triterpenoid-type saponins being predominant in cultivated crops. Some well-known triterpenoid saponin-accumulating plant families include the Leguminosae, Amaranthaceae, Apiaceae, Caryophyllaceae, Aquifoliaceae, Araliaceae, Cucurbitaceae, Berberidaceae, Chenopodiaceae, Myrsinaceae and Zygophyllaceae, among many others (Parente & Da Silva, 2009; Shi et al., 2004; Sparg et al., 2004). Legumes such as soybeans, beans and peas are a rich source of triterpenoid saponins. In contrast, cereals and grasses are generally deficient in saponins, with some notable exceptions such as Avena species (oats) which accumulate both triterpenoid and steroidal saponins (Osbourn, 2003). The steroidal saponins are typically found in members of the Agavaceae, Alliaceae, Asparagaceae, Dioscoreaceae, Liliaceae, Amaryllidaceae, Bromeliaceae, Palmae and Scrophulariaceae families and accumulate in abundance in crop plants such as yam, alliums, asparagus, fenugreek, yucca and ginseng (Hoffmann, 2003; Hostettmann & Marston, 2005). Diosgenin, the steroidal aglycone obtained by the hydrolysis of dioscin, a saponin abundant in the tubers of Dioscorea villosa (wild yam), is the precursor for the commercial synthesis of steroids such as cortisone, progesterone and pregnenolone (Patel et al., 2012; Peng et al., 2011). The steroidal glycoalkaloids are commonly found in members of the Solanaceae family including tomato, potato, aubergines and capsicum (Roddick & Melchers, 1985).
Several plants release saponins into the rhizosphere where these compounds might serve as allelopathic agents that suppress the growth of neighboring plants and prevent competition for natural resources. For instance, the germination, growth and yield of cotton was found to be lower when plants are grown on soil previously used for cultivation of Medicago sativa (alfalfa), compared with growth on soil after wheat cultivation or on uncultivated bare soil (Leshem & Levin, 1978). Similar effects were also observed for wheat. Alcoholic extracts of alfalfa roots suppressed germination of wheat seeds, and application of powdered alfalfa roots or compounds purified from alfalfa roots inhibited wheat seedling growth (Oleszek, 1993; Oleszek & Jurzysta, 1987). The antifungal triterpenoid saponins avenacins, which are synthesized in and released from oat roots, have also been suggested to have allelopathic potential, particularly because of the invasive nature of oat growth when compared to other cereals (Carter et al., 1999; Field et al., 2006; Hostettmann & Marston, 1995). A meta-transcriptomics-based analysis of bulk soil and rhizosphere of 4-week old pea, wheat and oat plants revealed major differences between their microbiomes. The relative abundance of nematodes and/or fungi was fivefold more in pea and oat rhizospheres than in the wheat rhizosphere and bulk soil. The pea rhizosphere was enriched for fungi, unlike the oat rhizosphere, which contained antifungal avenacins. Comparison of the rhizosphere microbiomes of oat wild-type and sad1 mutants (lacking avenacins) showed little differences in the prokaryotic community but large variation in the nematode and fungal community, suggesting a broader role for avenacins than just protecting from fungal pathogens (Turner et al., 2013).
In both monocotyledonous and dicotyledonous plants, the oleananes, one of the main classes of triterpenes derived from 2,3-oxidosqualene (Figure 1), represent the most common aglycone skeleton for the synthesis of triterpenoid saponins. The oleananes are pentacyclic triterpenoids that are found in almost all plant orders except the Solanales, Juglandales, Rhamnales and Zingiberales (Vincken et al., 2007). Although less abundant, the ursanes also occur in many plant families and are composed of a pentacyclic triterpene backbone that is almost identical to that of the oleanane backbone but differs in the location of one methyl group. The lupane and dammarane structures differ considerably from the oleananes and ursanes, and serve as aglycones for less common pentacyclic and tetracyclic saponins, respectively. The remaining classes of triterpenoid saponins, including the tirucallanes, hopanes, taraxasteranes, lanostanes and cucurbitanes are restricted to only a few plant families. The cycloartanes, also cyclized from 2,3-oxidosqualene, are an exception as they form the backbone for the synthesis of not only specialized metabolites (steroidal saponins and glycoalkaloids) but also phytosterols, and are present in all plants (Vincken et al., 2007). Furthermore, the phytosterols cholesterol, campesterol and sitosterol are the precursors of the brassinosteroid hormones that regulate plant growth and development (Fujioka & Yokota, 2003).
OSC genes encoding β-amyrin synthases for the cyclization of 2,3-oxidosqualene to the oleanane backbone β-amyrin have been identified in a number of different plant species (http://www.brenda-enzymes.org/php/result_flat.php4?ecno=5.4.99.39), including the model plant A. thaliana, which does not accumulate oleanane-derived saponins (Shibuya et al., 2009). In addition to β-amyrin, other simple triterpenes and triterpenoids have been detected as major components of the intracuticular wax layer in many plants (Buschhaus & Jetter, 2011). The widespread presence of simple triterpenes and triterpenoids in plants when compared with the taxon-specific accumulation of specialized glycosylated saponins in particular plant species suggests a possible fundamental role for these saponin biosynthetic precursors. These molecules may form constituents of plant membranes and may further have as yet unidentified signaling roles, given their structural similarities with sterols.
Saponins accumulate in an organ- and/or tissue-specific manner and the saponin content of plants is influenced by developmental factors, indicative of differential synthesis and regulation and suggesting distinct biological functions. Different types of saponins have been isolated from roots, stem, bark, leaves, seeds, fruits and flowers, and from whole plants (Vincken et al., 2007). In many plants, saponins are primarily synthesized and stored in underground organs. Glycyrrhiza glabra (licorice), Panax ginseng (ginseng) and the Chinese traditional medicine plant Polygala tenuifolia accumulate glycyrrhizin (G. glabra), ginsenosides (P. ginseng) and senegenin-derived saponins (P. tenuifolia) in the stolon, roots and/or rhizomes (Park et al., 2005; Seki et al., 2008; Teng et al., 2009). The roots of Bupleurum species and the model legume Medicago truncatula accumulate higher levels of saponins than the aerial organs (Huhman et al., 2005; Kapusta et al., 2005; Zhao et al., 2013). The total saponin content of M. truncatula roots is estimated at 5.92 µg/mg of dry weight, which is 5-, 10- and ∼200-times more than the saponin content of leaves and seeds, stems and seed pods, respectively (Huhman et al., 2005). Saponins can also accumulate in a tissue-specific manner in the roots of some plants. In P. ginseng, the ginsenosides are present in higher amounts in the phloem than the xylem, and are also found in parenchymal cells of the pith in the root (Fukuda et al., 2006; Yokota et al., 2011). In young Bupleurum roots, the saikosaponins are mainly in the pericycle and primary phloem, and in mature roots, they are distributed in the vascular cambium and secondary phloem (Tan et al., 2008). In oat roots, the UV-fluorescent saponin avenacin A-1 accumulates specifically in the epidermal layer of the root tips (Osbourn et al., 1994; Turner, 1960). However, saponins can also preferentially accumulate in aerial organs. Examples include the avenacoside steroidal saponins which are found in oat leaves, the asiaticosides in Centella asiatica leaves (Kim et al., 2005), the saponin tomatoside A in seeds of tomato (Moco et al., 2007), and saponins in flowers, fruits and seeds of Chenopodium quinoa (Kuljanabhagavad et al., 2008).
Simple triterpenes may also accumulate in a tissue- and organ-specific manner during plant growth. For example, in legumes, β-amyrin and lupeol have been detected in nodule tissues (Hartmann et al., 2002), and sprouting has been reported to increase both β-amyrin and saponin content of seedlings (Ayet et al., 1997; Baisted, 1971; Guajardo-Flores et al., 2012; Livingston et al., 1984). Likewise, in Solanum species, differential tissue- and organ-specific synthesis and accumulation of steroidal glycoalkaloids has been observed (Mweetwa et al., 2012). Synthesis commences during germination and peaks in the flowering period. The leaves are the first organs to attain maximum concentration, followed by unripe fruits and flowers which accumulate even higher amounts of the metabolites (Friedman, 2006). In potato tubers, the steroidal glycoalkaloids are concentrated in the peel with highest levels around the eyes constituting of the periderm, cortex and outer phloem (Friedman et al., 2003). The model plant A. thaliana has 13 OSCs which catalyze the cyclization of 2,3-oxidosqualene to different major triterpene products. These OSC genes differ in their organ-specific expression patterns, suggesting specific functions for the different triterpene products in different organs during different stages of plant growth and development (Thimmappa et al., 2014; Figure 2A).
Organ- and tissue-specific expression of oxidosqualene cyclase genes in Arabidopsis thaliana and Avena strigosa. (A) Heat map depicting the expression profiles for 12 of the 13 A. thaliana OSC genes in various plant organs. Transcripts for CAMS1 (At1g78970) were not detected and hence not included. Expression data were retrieved from Genevestigator V3. BARS1, baruol synthase; CAS1, cycloartenol synthase; LSS1, lanosterol synthase; LUP1, lupeol synthase; LUP2, β-amyrin synthase; LUP4, β-amyrin synthase; LUP5, tirucalla-7,21-dien-3β-ol synthase; MRN1, marneral synthase; PEN1, arabidiol synthase; PEN3, tirucalla-7,24-dien-3-ol synthase; PEN6, bauerenol synthase; THAS1, thalianol synthase. From Thimmappa et al. (2014). (B) Distribution of β-amyrin synthase (Sad1) and cycloartenol synthase (CS1) gene transcripts in the root tip of A. strigosa. In situ mRNA hybridization of young root tips shows Sad1 transcripts in the epidermis, weakly in parts of the subepidermis and some columella cells. CS1 expression is restricted to the cortex in the elongation zone above the root tip. From Wegel et al. (2009).
The isoprenoids for the synthesis of both sterols and specialized triterpenes are derived from the shared mevalonate pathway, and there is evidence for inverse regulation of the biosynthetic pathways for the synthesis of phytosterols and triterpenes in plants. During the early stages of pea germination, sterol synthesis is very low and β-amyrin production is predominant. During development, however, sterol synthesis is prevalent. In the late fruit maturation period, sterol synthesis decreases considerably and there is significant synthesis of β-amyrin (Baisted, 1971). Similarly, sorghum grains accumulate β-amyrin early in root development, while sterol biosynthesis becomes active only later (Palmer & Bowden, 1977). The end products of sterol and triterpenoid biosynthesis also manipulate the flux through these biosynthetic pathways (Haralampidis et al., 2001b). For example, feeding radiolabeled acetate to Gypsophila paniculata and Saponaria officinalis cell suspension cultures pre-treated with gypsogenin 3,O-glucuronide (a saponin precursor in G. paniculata, but not S. officinalis) resulted in reduced incorporation of radioactivity in saponins and their precursors, but not sterols in G. paniculata. Compared to untreated controls the ratio of β-amyrin to cycloartenol reduced by ∼10-fold in G. paniculata but remained unchanged in S. officinalis (Henry et al., 1992). Also, in cell suspension cultures of Tabernaemontana divaricate, inhibition of the activity of cycloartenol synthase resulted in the induction of β- and α-amyrin synthases, which in turn increased flux through the triterpenoid pathway (van der Heijden et al., 1989). In many cases, there is a good correlation between the accumulation of metabolites and the expression pattern of the respective biosynthetic genes. In Glycyrrhiza glabra seedlings, the expression of the β-amyrin synthase gene is maximal two days after germination and thereafter decreases rapidly, whereas the expression of the cycloartenol synthase gene remains relatively constant throughout germination (Hayashi et al., 2004). In oat roots, a marked difference is observed in the spatial expression patterns of the cycloartenol synthase and β-amyrin synthase genes, which are required for synthesis of sterols and triterpenoid saponins, respectively. In young oat roots, the β-amyrin synthase gene Sad1 is expressed in the epidermal cells of the root tips, in accordance with the accumulation of the β-amyrin derived avenacins. In contrast, expression of the cycloartenol synthase gene CS1 is restricted to the cortex in the elongation zone above the root tip (Figure 2B; Wegel et al., 2009).
Genotype- and cultivar-dependent variation in the saponin content of plants has also been reported. For instance, the grain saponin content of different cultivars of C. quinoa is estimated to range from 0 to ∼10 mg/g dry weight, and based on the total saponin content these cultivars are classified as bitter (high saponin) or sweet (low saponin) (Koziol, 1992). Between the two Centella species, C. asiatica and C. glabrata, the triterpenoid saponin asiaticoside and related compounds are major constituents in the former species and absent in the latter (Long et al., 2012). Similarly, a systematic analysis of the triterpene glycosides asiaticoside and madecassoside in sixty C. asiatica accessions from South India grown under identical environmental conditions revealed huge variations in the content of these saponins, with three accessions having no detectable asiaticoside/madecassoside (Thomas et al., 2010). Analysis of the saponin composition of 800 cultivated (Glycine max) and 329 wild (Glycine soja) soybean seeds showed clear variety-specific differences between the accessions. These differences were attributed to variety-specific expression of biosynthetic genes that utilize soyasapogenol glycosides as substrates (Tsukamoto et al., 1993). In G. max, the levels of group B soyasaponins represent one of the most discriminatory metabolic indicators of closely related salt-sensitive and salt-tolerant soybean genotypes (Wu et al., 2008). Large variation in the seed saponin composition of 3025 G. soja accessions from nine regions of Korea has also been reported. Based on the saponin composition of the seed hypocotyls, the accessions were grouped into seven phenotypes (Panneerselvam et al., 2013). In addition, comparison of six Cajanus cajan (chickpea) and five Phaseolus vulgaris (common bean) genotypes showed genotypic variations in their sapogenol content (Vasishtha & Srivastava, 2011, 2012).
In addition to genetic determinants of saponin content, environmental and agronomic factors may also influence the saponin composition of plants (Etebu, 2012; Schwarzbach et al., 2006). For example, potato tubers show pronounced drought- and light-induced accumulation of steroidal glycoalkaloids in their outer layers when stored in the light instead of the dark (Bejarano et al., 2000; Percival, 1999). In contrast, ginsenoside biosynthesis in P. ginseng is enhanced in the dark (Kim et al., 2014). Seasonal variation in the expression of the β-amyrin synthase gene and the content of glycyrrhizin has also been observed in G. glabra plants (Hayashi et al., 1998, 2004). The combination of environmental stress and agricultural factors such as water-logging or drought, fertilization and low or high temperatures influences the content of triterpenoids and saponins in plants. In the medicinal herb Hypericum brasiliense, betulinic acid content increases in response to drought and low or high temperatures (Nacif de Abreu & Mazzafera, 2005). Drought stress significantly increases the production of the triterpenoids rosmarinic, ursolic and oleanolic acid in spicas (reproductive parts) of Prunella vulgaris (Chen et al., 2011). Also in Bupleurum chinense, mild water stress increases the saikosaponin content of roots (Zhu et al., 2009).
The occurrence and distribution of saponins in plants in a tissue- and organ-specific manner at different growth and development stages suggests that these metabolites may have functions in physiological processes in plants, in addition to their traditionally accepted roles as phytoalexins and defense molecules.
Effects on plant growth and development
The ability of different plant species to synthesize distinct secondary metabolites is generally thought to be essential for their survival. Indeed, the saponin content and composition of a plant is variable and influenced by its surrounding environment and growth stage (Szakiel et al., 2011). Stress- or developmentally regulated changes in saponin composition contribute to the total plant secondary metabolite pool where they may ensure protection of plant organs and overall plant survival. However, as described above, in many plants, saponins and their biosynthetic intermediates accumulate during normal growth and development. Manipulating the biosynthesis of these molecules causes morphological and physiological effects, suggesting additional functions (Abe et al., 1988; Guhling et al., 2006). In this section, we present growing evidence linking the saponins and their biosynthetic intermediates to various physiological processes during plant growth, development and maturation (Figures 3 and and4).4). We also highlight the impact of modulating saponin synthesis and accumulation in plants and suggest their underexplored primary role in plant well-being.
Plant growth and development effects upon external application of saponins and their intermediates. (A) Effect of application of chromosaponin I (CSI, isolated from Pisum sativum) on Arabidopsis thaliana root growth. Root length, root cell number and length of mature cells increase in both Landsberg erecta (Ler) and Columbia (Col) wild-type (WT) ecotypes upon CSI application. However, the root diameter decreases upon CSI treatment in both ecotypes. From Rahman et al. (2000). (B) Effect of application of 0.018% betulin or its glucosides on Medicago sativa radicle and hypocotyl growth. No effects were observed on hypocotyl growth, but a clear inhibitory effect was seen on radicle growth with increasing number of glucosides attached to betulin. From Ohara & Ohira (2003). (C) Inhibitory effect of application of α-tomatine on stem elongation and chlorophyll accumulation in Sesbania exaltata, Senna obtusifolia, Vigna radiata, Triticum aestivum and Sorghum vulgare. From Hoagland (2009). The structures of the applied compounds are given. Gal, galactose, Glc, glucose, GlcA, glucuronic acid; Rha, rhamnose; Xyl, xylose.
Plant growth and development effects resulting from altered in planta saponin biosynthesis. Various developmental effects resulting from the over or reduced accumulation of saponins and/or their intermediates is depicted in Solanum lycopersicum, Medicago truncatula, Arabidopsis thaliana, Lotus japonicus and Avena strigosa. Gene names in upper and lower cases imply gene overexpression and knock out, respectively.
Effects of exogenous application
The external application of saponins to plants impacts different biological processes (Geuns, 1978). Seeds of pea and corn absorb water more rapidly in the presence of saponins, leading to more rapid germination. Crude saponin extracts from Quillaja, Polygala, Saponaria and Sapindus species can double the growth rate of isolated wheat and pea embryos (Helmkamp & Bonner, 1953). Asiaticosides extracted from Centella asiatica stimulate growth and chlorophyll biosynthesis in radish, pea, Lupinus and Linum species (Boiteau & Ratsimamanga, 1956). Saponins from pea have been proposed to regulate gravitropism and cellulose synthesis in different plant species (Ohana et al., 1998; Rahman et al., 2001). Chromosaponin I, isolated from 7-day-old etiolated Pisum sativum seedlings, stimulates cortical cell division and elongation leading to enhanced growth of roots in A. thaliana, Lactuca sativa, Chrysanthemum coronarium, Medicago sativa, Trifolium repens, Astragalus sinicus, Brassica juncea, Brassica campestris, Cryptotaenia japonica, Phleum pretense and Lolium multiflorum (Rahman et al., 2000; Tsurumi & Wada, 1995). In addition to enhanced elongation of roots, a reduction in root diameter was also observed in A. thaliana (Figure 3A) and L. sativa, suggesting a potential growth regulatory role for chromosaponin I, which appears to be modulated by the auxin influx carrier protein AUX1 (Rahman & Tsurumi, 2002; Rahman et al., 2001). In contrast, betulin and its glycosides show growth inhibitory effects on alfalfa radicles, with the inhibitory activity tending to increase with an increase in the number of β-d-glucose residues. The hypocotyls were less affected than the radicles for all tested betulin derivatives, suggesting that these compounds primarily affect the roots (Figure 3B). In the absence of sugar molecules, the triterpenoid betulinic acid, with a carboxyl group at carbon position 28, showed stronger inhibitory activity than betulin, which has a hydroxyl group at the same position. Oleanolic acid, also with a carboxyl group on position 28, but having an oleanane instead of a lupane backbone (Figure 8), showed no growth effects on alfalfa radicles or hypocotyls (Ohara & Ohira, 2003).
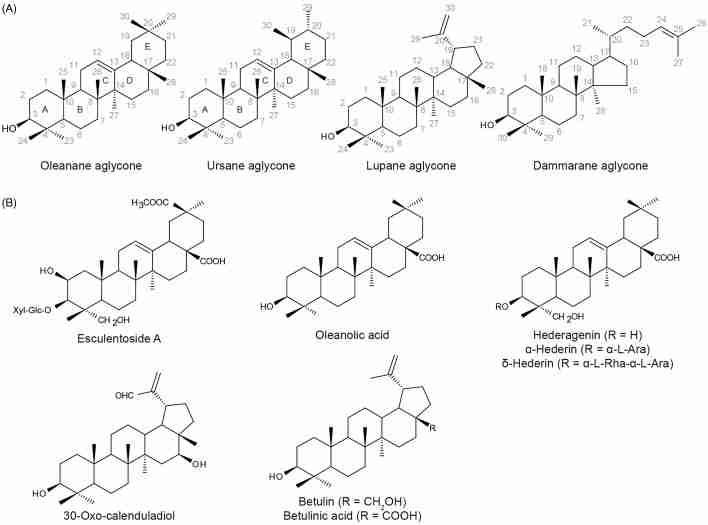
Overview of chemical structures. (A) Carbon and ring numbering of oleanane, ursane, lupane and dammarane aglycones. (B) Examples of triterpenoids and saponins discussed in structure-activity relationship studies. α-l-Ara, α-l-arabinose; α-l-Rha, α-l-rhamnose; Glc, glucose; Xyl, xylose.
In contrast to the effects observed with triterpenoid saponins, which mainly affect the roots, the exogenous application of the steroidal glycoalkaloid saponin α-tomatine inhibits stem elongation and chlorophyll accumulation in seedlings of Sesbania exaltata, Senna obtusifolia, Vigna radiata, Triticum aestivum and Sorghum vulgare (Figure 3C). α-Tomatine also causes electrolyte leakage from leaf discs of Zea mays, Cucumis sativus, Nicotiana tabacum and Nicandra physaloides, but not from tomato and potato tissues (Hoagland, 2009). In comparison, the aglycone tomatidine causes greater electrolyte leakage in Z. mays, C. sativus, N. tabacum and N. physaloides. Application of pure tomatidine to A. thaliana seedlings caused root growth effects without affecting their germination rate. The roots of seedlings grown on medium supplemented with tomatidine were shorter than those of seedlings grown on control medium or medium supplemented with α-tomatine (Itkin et al., 2011).
Effects of altering the endogenous levels of simple triterpenes
Growth and development
Modulation of the expression of genes encoding the OSCs that generate triterpene backbones has revealed that simple triterpenes can effect plant growth and development. Arabidopsis thaliana seedlings overexpressing thalianol synthase (THAS) gene, which encodes a protein for the cyclization of 2,3-oxidosqualene to the specialized triterpene thalianol, show stunted growth but have longer roots compared to wild type seedlings (Field & Osbourn, 2008). Similarly, lack or overaccumulation of marneral and/or its metabolites incur detrimental effects on plant growth and development in A. thaliana (Field et al., 2011; Figure 4). Mutants for the OSC marneral synthase (MRN1), which cyclizes 2,3-oxidosqualene to marneral in A. thaliana, show delayed embryogenesis, late flowering, rounded leaves, aberrant seed morphology, reduced growth caused by inhibition of cell expansion and elongation and defects in membrane integrity and permeability. Interestingly, these effects could not be attributed to the altered expression of genes involved in cell expansion, while it is unknown whether marneral and its derivatives serve as membrane constituents. At the transcriptional level, these compounds have been proposed to have a negative role in flower development, since the delayed flowering phenotype in the mrn1 knockout mutant could be attributed to increased expression of the flowering locus C (FLC) gene that encodes a repressor of flowering in A. thaliana (Go et al., 2012).
Simple triterpenoids are also essential constituents of the cuticles that cover plant aerial surfaces (Brendolise et al., 2011; Guhling et al., 2005; Huang et al., 2012). The cuticle is composed of two main components – very long chain fatty acid-derived cutin; and waxes, which are mixtures of primary alcohols, aldehydes and triterpenoid alcohols (Jetter et al., 2000). The wax is both epicuticular and intracuticular, forming a continuous layer on the top of the cutin and also being embedded in it. Triterpenoids are dominant constituents of the intracuticular wax (Buschhaus & Jetter, 2011; Buschhaus et al., 2007; Van Maarseveen & Jetter, 2009). In A. thaliana, the GLABROUS1 (GL1) gene is required for the development of specialized trichome cells. The A. thaliana gl1 mutant is trichome-less and lacks triterpenoids in its wax layer. Ectopic expression of AtLUP4, a β-amyrin synthase gene, in the gl1 mutant results in the accumulation of β-amyrin in the intracuticular wax layer of A. thaliana (Buschhaus & Jetter, 2012). This accumulation of β-amyrin in the gl1 mutant as a consequence of the expression of AtLUP4 under the control of a double constitutive cauliflower mosaic virus 35S promoter caused a significant reduction in water barrier effectiveness and thereby reduced intracuticular wax resistance.
In oats, increased β-amyrin content in the roots has recently been shown to result in shorter roots with a super-hairy phenotype (Kemen et al., 2014). sad2 mutants of diploid oat (A. strigosa) are blocked in the second step in the avenacin pathway (the oxidation of the β-amyrin scaffold; Geisler et al., 2013) and so accumulate elevated levels of this triterpene. Accumulation of high levels of β-amyrin triggers a change in cell fate in the root epidermis, with more cells being specified as trichoblasts (cells that give rise to root hairs; Kemen et al., 2014). This is the first demonstration of a role for a simple triterpene in plant development by changing cell fate in the root. A clear link between root length and the amount of β-amyrin accumulating in the roots was also observed, indicating that accumulation of elevated levels of β-amyrin triggers changes in cell specification and root length (Figure 4).
Nodulation
Lupane-type triterpenes have been proposed to play important roles as hydrophobic and antibiotic barriers in the development of symbiosis in legumes (Hayashi et al., 2004). During the establishment of symbiotic relations with rhizobia bacteria or mycorrhizal fungi, the synthesis of the ursane- and oleanane-type triterpenes α- and β-amyrin is also up-regulated (Baisted, 1971; Grandmougin-Ferjani et al., 1999; Hernández & Cooke, 1996; Iturbe-Ormaetxe et al., 2003). The expression of the native β-amyrin synthase is high in root nodules of G. glabra (Hayashi et al., 2004) and pea seedlings infected with Rhizobium leguminosarum (Iturbe-Ormaetxe et al., 2003). From an analysis of P. sativum nodules, Hernández & Cooke (1996) detected β-amyrin in peribacteroid membranes and the microsomal fraction of nodule cells. β-Amyrin was not detected in free-living bacteria or in the plasma membrane of the roots, suggesting that this compound is of host-plant origin (Hernández & Cooke, 1996). About 90% of the Vicia faba nodule outer cortex tissue is composed of triterpenoids. Of the 90%, over 82% is betulin while the remaining 8% is lupeol (Hartmann et al., 2002). The ectopic expression of AsOXA1 (a β-amyrin synthase from Aster sedifolius) in M. truncatula resulted in the accumulation of significantly higher levels of β-amyrin-derived sapogenins in the leaves, roots and nodules of the transgenic plant, which exhibited a high-nodulating phenotype (Confalonieri et al., 2009; Figure 4). Together, these reports suggest a yet undefined role for specialized simple triterpenes in the establishment and progress of nodulation in several legumes. However, more recent data indicate that simple triterpenes may have more complex roles in symbiosis.
The AMY2 gene, which encodes a multifunctional OSC that cyclizes 2,3-oxidosqualene to β-amyrin and dihydrolupeol, is highly expressed in the roots of Lotus japonicus and in nodules infected with the symbiotic bacterium Mesorhizobium loti. Silencing of AMY2 caused root growth and development defects but did not have any obvious effect on nodulation. Transgenic AMY2 silenced roots generated using Agrobacterium rhizogenes-mediated transformation led to severe retardation of root growth, while stably silenced plants had short stunted roots (Krokida et al., 2013). Expression of the gene for another L. japonicus OSC, OSC3 (a lupeol synthase) is induced upon nodulation and is localized to the vascular bundles, inner cortical cells and uninfected cells of the central tissue of developing and mature nodules upon infection with M. loti. The expression pattern of the OSC3 gene may suggest a structural role for lupeol in the membrane of developing nodules and/or a role in transportation of compounds in and out of the nodules (Delis et al., 2011). Silencing of OSC3 in L. japonicus abolished lupeol synthesis and resulted in significantly more visible nodules compared to the control plants 20 days post inoculation with M. loti (Figure 4). In M. truncatula and G. glabra, the lupeol synthase homologs are also expressed during nodule formation, suggesting a general role for lupeol in nodulation across leguminous species (Hayashi et al., 2004; Gamas et al., 1996; Shibuya et al., 1999). In the OSC3 silenced L. japonicus roots, the absence of lupeol resulted in a rapid nodulation phenotype and was accompanied by the elevated expression of early nodulin 40 (ENOD40), an early marker gene for nodule primordia initiation and cortical cell division. Exogenous application of lupeol to M. loti-infected wild-type plants provided further evidence for a negative regulatory effect of lupeol on the expression of ENOD40 (Delis et al., 2011). Through a series of in vitro screens lupeol has also been shown to activate apoptosis in human cancer cells by decreasing the β-catenin protein level and inducing degradation in the β-catenin signaling pathway (Saleem et al., 2009). Homologs of the mammalian β-catenin gene have been identified in plants and like in mammals the β-catenin protein in plants localize to the nucleus or cytosol depending on the availability of interacting proteins (Coates, 2003). Although the mode of action of the β-catenin proteins in plants has not been entirely characterized, associated proteins have been identified in gibberellin and brassinosteroid signaling pathways (Amador et al., 2001; He et al., 2002; Lee et al., 2002). Also in A. thaliana, β-catenin related proteins have been identified and shown to promote lateral root development (Coates et al., 2006). However, a role for β-catenin and related proteins in nodulation has not yet been reported.
Considering the structural similarity of triterpenes and triterpenoids with primary sterols, these specialized compounds have been proposed to function as membrane constituents. However, very little is known about the temporal and spatial embedding of these molecules in the membranes or the role that they may play during normal plant growth. Triterpenes may substitute sterols at certain developmental stages as has been suggested for marneral (Go et al., 2012), or they may have functions in various physiological processes such as nodule formation and cuticle composition. It has also been suggested that isoprenoids, unidentified as yet but other than sterols, are responsible for the activity of plant microRNAs (miRNAs) that guide ARGONAUTE (AGO) protein complexes to regulate expression of complementary RNAs. In the A. thaliana mad3 mutant, which has a miRNA activity deficient phenotype, the functions of AGO1 are compromised due to its reduced membrane association. This membrane perturbation was suggested as the underlying mechanism for the miRNA deficiency (Brodersen et al., 2012). It is intriguing to consider that the presence of specialized triterpenes in plant membranes, and possibly also the membrane permeabilizing properties of saponins, may be implicated in these phenotypes.
Effects of accumulating other saponin pathway intermediates
Mutation of genes encoding P450s and/or UGTs involved in saponin biosynthesis in M. truncatula and S. lycopersicum has revealed detrimental effects on plant growth that may be attributable to the accumulation of toxic intermediates. The following examples highlight the need for full glycosylation of saponins in order to maintain normal plant architecture (Figure 4). M. truncatula synthesizes a diverse array of saponins that can be classified into two main types, the hemolytic and non-hemolytic saponins, based on their ability to lyse red blood cells. Both types of saponin are synthesized through sequential oxidation and glycosylation of the oleanane aglycone, β-amyrin. The nature of the P450-mediated oxidation of the aglycone determines whether the saponins are hemolytic or non-hemolytic. The multistep oxidation of carbon 28 by the P450 CYP716A12 is essential for the synthesis of hemolytic saponins. Following this oxidation event, a glucose moiety is added at this position by the glucosyltransferase UGT73F3. The M. truncatula loss-of-function mutants of CYP716A12 and UGT73F3 do not accumulate hemolytic saponins and have a dwarf phenotype (Carelli et al., 2011; Naoumkina et al., 2010). The UGT73F3 mutants flowered and generated pods with morphologically normal seeds that had a reduced germination rate (Naoumkina et al., 2010). A similar, strong retarded growth phenotype is also observed in tomato plants upon silencing of the GlycoAlkaloid MEtabolism 1 (GAME1) gene, a galactosyltransferase involved in the synthesis of the steroidal glycoalkaloid α-tomatine. The GAME1-silenced plants accumulate ∼300-fold more tomatidine than wild-type plants, develop deformed leaves and produce many small flower buds, most of which are aborted before fertilization, resulting in the development of a small number of parthenocarpic, shriveled fruits (Itkin et al., 2011).
In A. strigosa and M. truncatula, full glycosylation of root saponins has been shown to be critical to prevent deleterious effects on plant root physiology (Figure 5). Mutants of A. strigosa that are affected at either the Sad3 or the Sad4 locus (both of which are as yet uncloned) accumulate avenacin A-1 lacking the β-1,4-linked d-glucose moiety. Both types of mutants have reduced root growth and reduced numbers of root hairs, root epidermis-specific membrane trafficking defects, and accumulate callose (a plant polysaccharide composed of glucose residues). As callose deposition can be induced in plants upon pathogen attack or wounding, its accumulation in the sad3 and sad4 mutants is likely to be a stress response caused by toxicity of the incompletely glucosylated intermediate. The sad3 and sad4 root phenotypes are suppressed in a sad1 mutant background, consistent with this; sad1/sad1 sad3/sad3 and sad1/sad1 sad4/sad4 double mutants do not accumulate avenacin intermediates and have normal roots, confirming that the accumulation of incompletely glycosylated avenacin biosynthetic intermediates is responsible for the cytotoxic effects observed in the roots (Mylona et al., 2008).
Phenotypic similarity of Medicago trunctula and Avena strigosa mutants accumulating incompletely glycosylated saponins. (A) Chemical structures of a representative monoglycosylated saponin, 3-O-Glc-Medicagenic acid accumulating in Mkb1 silenced M. truncatula roots (left) and avenacin A-1 lacking the β-1,4-linked d-glucose (marked with a red line) accumulating in sad3 and sad4 oat mutants (right). (B) Confocal microscopy analysis (left) of control (CTR, top) and Mkb1 silenced (bottom) M. trunctula hairy roots, compared to bright field (left) and UV illumination (right) microscopy of wild type (WT), sad3 and sad4 oat mutants (right) show similar root cap and root epidermal layer defects in both plants. From Mylona et al. (2008) and Pollier et al. (2013).
In M. truncatula the silencing of Makibishi 1 (Mkb1) gene encoding an E3 ubiquitin ligase involved in controlling the activity of HMGR (the rate-limiting enzyme of the MVA pathway) results in accumulation of monoglycosylated saponins in transgenic hairy roots. The cells in the transgenic hairy roots had an irregular shape instead of the normal cylindrical shape and lacked intercellular space, which caused severe cell enlacing rendering the tissue in the root cortex zone rigid and prone to tissue rupturing. As a result, the hairy roots were dissociated from each other and formed caltrop-like structures. Mkb1 recruits the endoplasmic reticulum-associated degradation (ERAD) protein quality control system to regulate the levels and activity of HMGR and hence the amount of saponin precursors generated via the MVA pathway. This mechanism prevents the unrestrained biosynthesis of saponins in M. truncatula. Therefore, upon silencing of Mkb1 the flux through the saponin biosynthesis pathway is disturbed, leading to an over-accumulation of monoglycosylated saponins and severe root morphology defects. The transgenic hairy root phenotype could be mimicked by the overexpression of the catalytic domain of HMGR, which does not undergo ERAD control and therefore allows unrestrained synthesis of saponins in M. truncatula. These transgenic hairy roots also accumulated monoglycosylated saponins and showed the caltrop-like hairy root morphology seen in Mkb1-silenced hairy roots, indicating that the incompletely glycosylated saponins are associated with the observed root morphology effects (Pollier et al., 2013).
The A. strigosa sad3 and sad4 mutants and the M. truncatula Mkb1-silenced mutant described above accumulate incompletely glycosylated saponin biosynthetic intermediates and show strikingly similar root phenotypes (Figure 5B). In the mutants of both A. strigosa and M. truncatula, the root epidermal cell layer was incompletely formed exposing the inner root cortical cells to the growth environment. The morphological damage to the roots caused leakage of intracellular compounds and release of incompletely glycosylated saponin intermediates. In contrast to growth medium of control hairy roots that did not contain saponins, the growth medium of the MKb1-silenced M. truncatula roots accumulated monoglycosylated saponins and its application to control hairy roots caused transient tissue loosening and mimicked the phenotype of the Mkb1-silenced hairy roots, suggesting toxic effects of incompletely glycosylated saponins on plant cells (Pollier et al., 2013).
The severe growth and development effects observed in plants when saponin glycosyltransferases are impaired indicates that complete glycosylation of saponin intermediates is important in protecting the plant from the toxic effects of accumulation of incompletely glycosylated compounds. For instance, the avenacosides accumulate in a biologically inactive form in oat leaves and are activated by deglycosylation upon tissue damage or pathogen attack (Gus-Mayer et al., 1994; Nisius, 1988). For other saponins, glycosylation is essential for membrane permeabilization and antimicrobial activity, which is lost upon removal of the sugar residues (Morrissey & Osbourn, 1999). For example, the ability of the fungal pathogen Gaeumannomyces graminis var. avenae to infect oat roots is dependent on its ability to produce a fungal avenacin hydrolase (Bowyer et al., 1995). Similarly, the ability to degrade the tomato steroidal glycoalkaloid α-tomatine contributes to the pathogenicity of various fungal pathogens of tomato (Morrissey & Osbourn, 1999; Ökmen et al., 2013; Pareja-Jaime et al., 2008; Sandrock & VanEtten, 2001). These α-tomatine detoxification mechanisms commonly involve removal of sugars from α-tomatine to partially glycosylated molecules such as β2-tomatine, or to the aglycone, tomatidine (Bednarek & Osbourn, 2009; Morrissey & Osbourn, 1999; Sandrock & Vanetten, 1998). α-Tomatine degradation products have also been reported to suppress induced defense responses in plants, suggesting that these molecules may have signaling properties. The tomato pathogen Septoria lycopersici produces a tomatinase enzyme that hydrolyses a single sugar from the tetrasaccharide chain of α-tomatine to give β2-tomatine. This hydrolysis product suppresses induced plant defenses, suggesting that the host plant has subverted this fungal detoxification mechanism to bolster its own defenses (Bednarek & Osbourn, 2009; Bouarab et al., 2002; Ito et al., 2004). It has also been reported that the aglycone tomatidine suppresses induced defenses in tomato cell cultures (Ito et al., 2004). Ökmen et al. (2013) tested the effects of α-tomatine and tomatidine on tomato cells and did not observe suppression of induced defense responses. They did not, however, test the effects of β2-tomatine. The effects of steroidal alkaloids on plant cells require further investigation. Of note, these molecules are known to interfere with regulation of sterol homeostasis in yeast (Simons et al., 2006).
Industrial potential as bioactive principles
With the broad diversity in structures and biological activities of saponins and their biosynthesis intermediates, these compounds have been sought for various applications in the pharmaceutical, cosmetic, food and agronomic industry (Augustin et al., 2011; Moses et al., 2013; Sparg et al., 2004). Saponins from Camellia oleifera and Sapindus mukorossi are useful in detergents and cosmetics as they have excellent foaming properties, moderate detergency and anti-dermatophytic activity (Chen et al., 2010; Tamura et al., 2012; Yang et al., 2010). Quil A, a mixture of over 25 different saponins extracted from Quillaja saponaria, is the most widely used saponin adjuvant in veterinary vaccines (Dalsgaard, 1978). Yucca schidigera and Q. saponaria extracts are used as livestock dietary additives for fecal odor control (Cheeke, 2009; Killeen et al., 1998). Several C-27 steroidal saponins show antifungal activity (Morrissey & Osbourn, 1999; Yang et al., 2006), and glycyrrhizin accumulating in licorice roots is an efficient natural sweetener (Kitagawa, 2002). The wide spectrum of biological activities of this specialized class of compounds has been attributed to the diverse array of structures synthesized naturally by plants. Although such structural diversity is beneficial, the vast spectrum of biological activities can prove to be antagonistic to their application, particularly in the pharmaceutical sector. For instance, many saponins are cytotoxic and therefore potential chemotherapeutic agents, but also have hemolytic activity hindering their application as anti-cancer drugs (Podolak et al., 2010).
Several studies have hence focused on generating semi-synthetic derivatives inspired by the naturally accumulating saponins and their biosynthetic intermediates to generate a new range of candidate drugs with enhanced activity and reduced adverse effects. For example, synthetic derivatives of the pentacyclic triterpenoids oleanolic, ursolic and betulinic acid, which naturally accumulate to high concentrations in plants, have been evaluated to be strikingly more active than the parent compounds in several clinical applications (Liby & Sporn, 2012; Liby et al., 2007a,b). The semi-synthetic derivatives of oleanolic acid (Figure 6), bardoxolone (CDDO) and bardoxolone methyl (CDDO-Me) were clinically evaluated as anti-cancer agents (Hong et al., 2012; Tsao et al., 2010). CDDO-Me was also clinically tested for anti-inflammatory activity in patients with chronic kidney disease and type 2 diabetes to improve kidney function (Pergola et al., 2011). However, the worldwide phase III trial of the drug was terminated following severe adverse effects and mortality in patients administered the drug (http://www.clinicaltrials.gov/show/{"type":"clinical-trial","attrs":{"text":"NCT01351675","term_id":"NCT01351675"}}NCT01351675). It has been speculated that CDDO and its derivatives interact with multiple targets or entire networks in the cell, and therefore might be most effective in the early stages of disease progression prior to irreversible tissue damage, rather than at advanced stages of disease (Liby & Sporn, 2012; Sporn et al., 2007). The patients in the phase III trial with CDDO-Me had moderate-to-severe chronic kidney disease with type 2 diabetes. A detailed analysis of data collected during this terminated trial can provide essential insight into the relation between adverse effect, mortality and dose of CCDO-Me given to patients, which can then be utilized for future tests with other CDDO analogues (Abboud, 2013). The imidazolide and ethylamide derivatives of bardoxolone, CDDO-Im and CDDO-EA, respectively, induce chondrogenic differentiation in organ cultures and could be used for the treatment of diseases where enhancing chondrogenesis is therapeutically useful, including the prevention or treatment of osteoarthritis (Suh et al., 2012). Multiple in vitro and in vivo studies with CDDO-Me have also demonstrated the effect of this compound in the prevention and treatment of solid tumors (Wang et al., 2014). The combination of CDDO-Me or CDDO-EA with vorinostat, a histone deacetylase inhibitor, effectively delayed tumorigenesis in mouse models, proving to be a promising combination for chemoprevention (Tran et al., 2013). Another semi-synthetic derivative evaluated clinically in phase I and II trials is bevirimat (Figure 6). This betulinic acid derivative is a potent inhibitor of human immunodeficiency virus (HIV) maturation, which showed promising clinical outcomes (Smith et al., 2007). Other derivatives of betulin, betulinic acid and dihydrobetulin also have demonstrated potent anti-HIV and anti-leishmanial activities (Sousa et al., 2014; Xiong et al., 2010).
Naturally, accumulating plant compounds also have biological activities of interest for clinical applications and are routinely evaluated for potential novel therapeutics. YQ2, a 6:4 (v:v) blend of partially purified Y. schidigera and Q. saponaria extracts, has been clinically studied as an anti-obesity medicine. Administration of YQ2 to 51 hypercholesterolemic patients showed a decrease in total and low-density lipoprotein cholesterol levels in blood plasma, compared to 36 patients given a placebo treatment. YQ2 also improved gastrointestinal motility in the treated patient group, thereby enhancing gastrointestinal functions (Kim et al., 2003). The triterpenoid saponin escin (Figure 6), isolated from seeds of Aesculus hippocastanum (horse chestnut), has been clinically evaluated in gel formulations as combination therapy with diethylammonium salicylate and heparin. The gel combination of the three active principles showed positive effects on tenderness reaction, edema formation and joint function, compared to placebo. This randomized, double blind, controlled, group comparative study with 158 patients, topically treated or not with escin, concluded that the gel combination containing escin was well tolerated and led to rapid pain reduction, implicating its recommended use for the treatment of blunt impact injuries (Wetzel et al., 2002). Celastrol (Figure 6), extracted from roots of the traditional Chinese herb Tripterygium wilfordii, has potent anti-inflammatory and anti-cancer activities, and holds great potential for the treatment of multiple myelomas and other hematological malignancies (Chiang et al., 2014; Kannaiyan et al., 2011). QS-21 (Figure 6), is a soluble fraction of triterpenoid saponins from Q. saponaria with immunological adjuvant activity. QS-21 is currently being clinically evaluated in phase I and II studies for use in human vaccines against HIV (Leroux-Roels et al., 2014), Mycobacterium tuberculosis (Montoya et al., 2013), varicella-zoster virus (Lal et al., 2013), cancer (Garçon & Van Mechelen, 2011) and malaria (Ndungu et al., 2012).
Despite the various potential applications of saponins, their biosynthetic intermediates and derivatives, very few compounds are currently commercially exploited. A major concern hindering their widespread application as therapeutics is the lack of information about the mechanisms of adsorption, distribution, metabolism, excretion and toxicity of these compounds in human and animal cells. Well-designed, associated, systematic in vitro and in vivo studies will play a key role in deepening our understanding of the mechanisms involved in the action of saponins, before their widespread usage as drugs in the future.
Exploring structure–activity relationships
Saponins, their biosynthetic intermediates and derivatives have been ascribed a number of pharmacological activities (Figure 7), most notably permeabilization of cell membranes, cytotoxic effects on cancerous cells, adjuvant properties, immunomodulatory potential and lowering of serum cholesterol (Francis et al., 2002; Hostettmann & Marston, 2005; Man et al., 2010; Sjölander et al., 1998; Sun et al., 2009). Despite their structural diversity these compounds share distinct biological properties, and therefore several studies have focused on understanding the various biological activities associated with them in relation to their structural features. Identification of effective combinations of key functional entities and aglycones responsible for specific bioactivities allows targeted synthesis of novel active principles with enhanced efficacy and reduced adverse effects. Here, we summarize some of the most investigated structure–activity relationships of these compounds.
Overview of biological activities of triterpenoids and saponins in animals. The structures of representative compounds for each activity are given, together with the various molecular targets and affected pathways. Gene, pathway or metabolite activation and inhibition are indicated by arrow heads and blunt ends, respectively. Akt, serine/threonine-specific protein kinase; Bcl2, B-cell lymphoma 2; bFGF, basic fibroblast growth factor; cIAP, cellular inhibitor of apoptosis; COX-2, cyclooxygenase-2; CTL, cytotoxic T-lymphocyte; CXCR4/CXCL12, C-X-C chemokine receptor type 4 complex with C-X-C motif chemokine 12; DR, death receptor; EGFR, epidermal growth factor receptor; ICAM-1, intercellular adhesion molecule 1; IFN-γ, interferon γ; IgG, immunoglobulin G; IKK, IκB kinase; IL-2, interleukin 2; i-NOS, inducible nitric oxide synthase; MMP9, matrix metalloproteinase 9; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2-ARE, nuclear factor (erythroid-derived 2)-like 2, antioxidant responsive element; PDGF, platelet-derived growth factor; PPARγ, peroxisome proliferator-activated receptor-γ; STAT3, signal transducer and activator of transcription 3; Th1, T helper 1; VEGF, vascular endothelial growth factor.
Effect on cell membranes
Several biological effects of saponins are attributable to their ability to permeabilize cell membranes. The hemolytic activity of saponins is a consequence of the formation of stable pores in the erythrocyte membrane (Seeman, 1967). The interaction of saponins with cholesterol in the lipid membrane leads to the formation of insoluble complexes, which rearranges the architecture of the cholesterol-containing lipid bilayer to generate pores and increase membrane permeability (Augustin et al., 2011; Lorent et al., 2014; Morrissey & Osbourn, 1999). The membrane permeabilizing activity of saponins is correlated with their amphipathic nature and affected by the aglycone structure, saccharide chain length, sugar variants in the glycosidic chain and type of aglycone–sugar and sugar–sugar linkages.
Among the various saponin structures, there is a clear association between the type of aglycone backbone and hemolytic activity. Saponins derived from the lupane aglycone do not permeabilize membranes and are only slightly hemolytic, compared to those derived from the oleanane aglycone which strongly permeabilize membranes and hemolyse red blood cells (Figure 8A; Gauthier et al., 2009a,b). However, not all oleanane-type triterpenoid saponins have hemolytic properties. In M. truncatula two distinct groups of oleanane-derived triterpenoid saponins have been described; the non-hemolytic soyasaponins and the hemolytic saponins derived from oleanolic acid (Oleszek, 1996; Yoshiki et al., 1998). The hemolytic saponins have a carboxyl group at position 28 of the oleanane backbone and other oxidations on carbons 2, 16 and/or 23, while the non-hemolytic soyasaponins have a hydroxyl group at carbon 24 and additional oxidations on carbons 21 and/or 22 (Figure 8A). Modification of the carboxyl group at position 28 through chemical addition of amide-containing aromatic rings significantly lowers hemolytic activity of the saponin, as demonstrated for esculentoside A (Figure 8B, an oleanane saponin isolated from roots of Phytolacca esculenta) compared with the non-modified parent saponin (Wu et al., 2007). As described above, targeted silencing of CYP716A12 encoding the P450 that catalyzes the C-28 carboxylation of M. truncatula saponins, results in transgenic plants of which the saponin extracts have lost their hemolytic activity, in contrast to wild type M. truncatula plant extracts which are highly hemolytic (Carelli et al., 2011). It has also been suggested that increasing the polarity in ring A of oleanane saponins (Figure 8A) by addition of a hydroxyl or carboxyl group together with maintaining relative hydrophobicity in rings D or E is essential for hemolytic activity (Voutquenne et al., 2002). Esterification of the carboxyl group at position 28 of hederagenin-derived saponins (Figure 8B), reduces polarity in rings D–E and increases hemolytic activity compared to its corresponding free acid (Chwalek et al., 2006).
The saccharide chain on the saponins has been extensively studied to establish correlations between chain length, sugar components and interglycosidic linkages that influence membrane-permeabilizing activity, as the sugar side chain is essential for the hemolytic activity of saponins. For both digitonin and avenacin A-1, sequential deglycosylation and sugar removal reduces hemolytic activity and decreases membrane permeability (Armah et al., 1999; Nishikawa et al., 1984). Comparison of two hederagenin-derived saponins α-hederin and δ-hederin (Figure 8B) has revealed that the former is more hemolytic than the latter, and this has been attributed to differences in the length of the glycosidic chain (Chwalek et al., 2006); δ-hederin has an α-l-rhamnose linked to an α-l-arabinose at carbon 3, while α-hederin has a single α-l-arabinose. The anomeric configuration of the linked sugars has also been demonstrated to influence the hemolytic activity of saponins. For instance, when the α-l-rhamnose is linked to a β-l-anomer of arabinose instead of the α-l-anomer like in δ-hederin, the hemolytic activity is considerably reduced in comparison to δ-hederin (Chwalek et al., 2006). It has been observed that in general the monodesmosidic saponins, which have sugar moieties linked to a single carbon on the aglycone, are more hemolytic than the bidesmosidic saponins with sugars linked at two different positions, usually carbon 3 and carbon 28, of the aglycone. Among the monodesmosidic saponins, those that are glycosylated at position 3 are often more hemolytic than those at position 28 (Voutquenne et al., 2002). The type of terminal sugar and the linkage between the saccharides of the saponin can also have considerable influence on hemolytic potential. For the diglycosidic saponins derived from hederagenin, increased activity was associated with β-configuration of the terminal sugar and 1→4 linkages between the sugars when compared with 1→6 linkages (Chwalek et al., 2006).
Cytotoxicity and anti-tumor activity
The cytotoxic activity of numerous triterpenes and their triterpenoid saponins has been exploited for anti-tumor application, and the ability of these compounds to induce apoptosis by different cellular mechanisms has been extensively investigated (Lee et al., 2011; Podolak et al., 2010; Raju & Mehta, 2009; Sun et al., 2006a; Thakur et al., 2011). The triterpenoid aglycones oleanolic acid and ursolic acid exhibit cytotoxicity against human leukemia and lymphoma cells, with the latter being more potent (Chiang et al., 2003). A series of derivatives of these triterpenes has been tested for the ability to inhibit nitric oxide production by macrophages, as potential anti-cancer and chemopreventive drugs (Favaloro et al., 2002; Finlay et al., 1997; Honda et al., 1997, 1999, 2000a,b, 2002). Several systematic structure-activity studies for these derivatives have led to some important general observations. The oleanane derivatives were found to be more potent than the corresponding ursane derivatives (Figure 8A). In ring A, enone functionality at position 3 of the aglycone significantly increases activity. A carboxyl, methoxycarbonyl, or nitrile group at position 2 also enhances activity, contrary to a hydroxyl, aminocarbonyl, methoxy, chloride or bromide groups, which decrease activity of the derivative. However, a formyl group at position 2 makes the derivative highly cytotoxic. The methyl groups at position 23 and 24 of the aglycone are important for nitric oxide inhibition activity, as their removal significantly decreases this activity. In the C ring, modifying the naturally occurring 12(13)-ene double bond to 9(11)-en-12-one, 12(13)-en-11-one, 13(18)-en-11-one or 12-one functionalities enhanced potency of the derivative, while a 9(11)-ene did not affect potency and saturation of ring C, 11(12),13(18)-diene or 9,11-epoxide functionalities were less potent. Among all the ring C modifications, the 9(11)-en-12-one functionality enhanced potency by 10–100 times and therefore was used for further modifications in ring A of the derivative. The presence of a carboxyl or nitrile group at position 2 and an enone functionality at position 3, synergistically enhanced potency ∼10 000 fold. Also the combination of 12(13)-en-11-one or 12(18)-en-11-one with enone at position 3 and nitrile at position 2 strongly enhanced potency. The clinically evaluated semi-synthetic derivative of oleanolic acid, CDDO (bardoxolone) was developed because of these meticulous structure–activity studies. CDDO contains the highly potent 9(11)-en-12-one functionality in ring C, a nitrile group at position 2, and an enone functionality at position 3 of the aglycone, and shows the strongest nitric oxide inhibitory activity among all the generated oleanolic acid derivatives. Preliminary structure–activity studies also indicate that derivatization of the carboxyl group at position 28 of β-hederin abolishes broad-spectrum antitumor activity, but increases antitumor selectivity and cytotoxicity (Liu et al., 2010).
Next to the oleananes and ursanes, the lupane- and dammarane-type triterpenoid saponins (Figure 8A) have been studied for their cytotoxic activity against various human cancer cell lines (Park et al., 2005; Podolak et al., 2010). Compared to hederagenin and oleanolic acid, the lupane triterpenoids betulin and betulinic acid showed higher cytotoxicity (Figure 8B; Gauthier et al., 2009a,b). Among the monodesmosidic lupane-type saponins, cytotoxicity was observed when α-l-rhamnose, α-d-mannose or α-d-arabinose sugar was linked to position 3, but not when β-d-glucose, or β-d-galactose was present at the same position. The bidesmosidic saponin of betulinic acid bearing α-l-rhamnose moieties at both the carbon positions 3 and 28 was more potent than the naturally occurring saponin containing α-l-arabinose at position 3 and β-d-glucose at position 28 (Gauthier et al., 2009a,b). The lupane-type saponins also show better cytotoxicity and stronger anti-cancer activity than the germanicane-type saponins (Thibeault et al., 2007). The lupane triterpenoid, dihydrobetulinic acid is a potent inhibitor of HIV replication. Structure–activity relationship studies have also suggested that the substitution at position 19 enhances the anti-HIV activity of the molecule, compared to betulinic acid (Fujioka et al., 1994). Also, the non-acid semi-synthetic lupane-type triterpenoid 30-oxo-calenduladiol (Figure 8B) shows potent anti-HIV activity. The aldehyde function at position 30 was found to be essential for the anti-HIV activity, as its absence resulted in an inert compound (Barroso-González et al., 2009). In contrast to most triterpenoid saponins, for the ginsenosides anti-cancer activity is inversely correlated with the number of linked sugars, as sugar moieties reduce the hydrophobicity of the compound and decrease membrane permeability (Qi et al., 2010). Also, reduced anti-cancer activity is observed with ginsenosides glycosylated at position 6 (Figure 8B), compared to those with sugars linked at position 3 or 20 (Chen et al., 2009; Li et al., 2009; Popovich & Kitts, 2002).
A major drawback in the clinical development of anti-cancer drugs, particularly those based on triterpenoid saponins, has been the hemolytic activity associated with these compounds. The oleanane triterpenoid saponins derived from hederagenin showed a strong correlation between hemolytic activity and cytotoxicity (Chwalek et al., 2006). For the steroidal saponins however, these activities are not correlated and it is suggested that, they unlike the triterpenoid saponins, execute hemolysis and cytotoxicity through distinct mechanisms (Wang et al., 2007). The structure–activity relationship studies for the steroidal saponins with respect to the variation in sugar chain and steroidal aglycone are extensively reviewed in Podolak et al. (2010).
Adjuvant properties
The ability of saponins to activate the mammalian immune system has led to significant interest in their potential as vaccine adjuvants. The most promising lead candidates are Quil A and its derivative QS-21 which are actively being evaluated in both human and animal vaccine formulations against HIV, cancer, malaria, various bacterial and viral infectious agents (Sun et al., 2009). However, the major drawbacks limiting their widespread application in human vaccines are the high toxicity, hemolytic properties and instability in aqueous solutions associated with saponins. Various structure–activity studies have therefore aimed at identifying the structural features that reduce undesirable effects and enhance or maintain their adjuvant activity. A comparative analysis of 47 saponins for adjuvant and hemolytic activities suggested that both the sugar chain and aglycone backbone are essential for adjuvant activity (Oda et al., 2000). The aldehyde functionality at position 4 of the QS-21 saponin (Figure 6) was found to be essential for antibody stimulation and induction of an immune response, as derivatives modified at this function did not show adjuvant activity (Soltysik et al., 1995). Using DS-1 a deacylated derivative of QS-21, the lipophilic acyl side chain was also shown to be essential for stimulating cytotoxic T-lymphocyte production against antigens, as the deacylated derivative only induced a weak immune response (Liu et al., 2002). Re-acylation at a different position than in QS-21 restored adjuvant activity to some extent, but like its parent DS-1 failed to induced cytotoxic T-lymphocyte production.
The soyasaponins and lablabosides also show strong adjuvant activity and low hemolytic activity, and contrary to the QS-21 do not contain acyl, carboxyl or aldehyde functionalities on their structure. On the other hand, the escins have an acyl group, but show poor adjuvant activity and high hemolytic activity making them poor adjuvant candidates (Oda et al., 2000). These observations highlight that the functionalities on the aglycone alone are not determinant factors for the adjuvant properties of the saponins, but the overall conformation harmoniously influence adjuvant activity. The soyasaponins with linked sugars were strong adjuvants, contrary to the corresponding soyasapogenol aglycones, which were inert. In addition, among the soyasaponins, the adjuvant activity generally increased with increase in sugar chain length (Oda et al., 2003). For the protopanaxadiol and protopanaxatriol-type saponins the number, position and type of sugar linkages did not affect adjuvant activity, but significantly influenced the nature of immune response (Sun et al., 2005, 2006b).
Molecular targets identified in bioassays
The simple triterpenoids, glycoalkaloids and saponins are multifaceted in their molecular mechanisms as they modulate multiple targets, including transcription factors and kinases, in multiple pathways (Figure 7). The activation of various pro-inflammatory mediators linked with different stages of cancer progression and inflammation like the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), signal transducer and activator of transcription 3 (STAT3) and serine/threonine-specific protein kinase (Akt), are down-regulated by natural and semi-synthetic triterpenoids, alongside the triterpenoid and steroidal saponins and steroidal glycoalkaloids (Kannaiyan et al., 2011; Laszczyk, 2009; Lee et al., 2011; Liby & Sporn, 2012; Pollier & Goossens, 2012; Raju & Mehta, 2009; Shanmugam et al., 2013; Wu et al., 2007; Yadav et al., 2010). The anti-inflammatory activity of the semi-synthetic triterpenoid CDDO and its derivatives is attributed to the ability of these compounds to inhibit the de novo synthesis of inducible nitric oxide synthase (i-NOS) and cyclooxygenase-2 (COX-2) in macrophages (Honda et al., 1997, 1999, 2000a,b, 2002). CDDO is an agonist of peroxisome proliferator-activated receptor-γ (PPARγ), which induces growth arrest and apoptosis in a variety of tumor cell types (Lapillonne et al., 2003; Tsao et al., 2010; Wang et al., 2000). The strongest anti-inflammatory and anti-carcinogenic activity induced by CDDO and its derivatives is the activation of the nuclear factor (erythroid-derived 2)-like 2 (NFE2L2 or Nrf2) – antioxidant responsive element (ARE) signaling cascade, which is a major regulator of the cellular anti-oxidant response (Liby et al., 2007b; Sporn et al., 2007).
The adjuvant properties of saponins to promote antibody response are mediated by their unique ability to stimulate both the T helper 1 (Th1) immune response and the production of cytotoxic T-lymphocytes (CTLs) (Sjölander et al., 1998; Sun et al., 2009). The simultaneous triggering of different immune responses makes them ideal for use in subunit vaccines, vaccines against intracellular pathogens and in therapeutic cancer vaccines.
Triterpenoids have also shown great promise for the treatment of HIV through their novel mechanism of action. The semi-synthetic triterpenoid bevirimat prevents the maturation of new virions and thus spread of the virus to new cells. During the Gag processing cascade, bevirimat specifically blocks the cleavage of the Gag capsid (CA) precursor CA-SP1 to mature CA protein, leading to defective core condensation and release of immature non-infectious virus particles (Li et al., 2003).
It is essential to emphasize the need for robust, systematic, well-designed studies to decipher the underlying mechanisms of the therapeutic action of saponins. In most structure–activity relationship investigations, the cell lines and animal models are randomly chosen and very few studies employ the same investigative models, making data comparison across studies futile. There are also a large number of studies with contradicting outcomes when in vitro and in vivo effects are compared; often questioning the relevance of structure–activity studies. However, to our opinion, the choice of cell lines and animal models partially account for some of these disconcerted results. Another key aspect to consider is the bioavailability of saponins in animal models, which is not addressed in in vitro investigations employing cell lines. Therefore, more studies are certainly needed to evaluate pharmacokinetics and bioavailability of saponins, their biosynthetic intermediates and derivatives.
Concluding remarks
Simple and elaborated triterpenoids affect fundamental cellular processes both in plants and animals. The molecular targets that are subject to the action of these compounds have not yet been identified in plants. Nevertheless, there is a remarkable functional conservation of groups of proteins that regulate cell proliferation, cell fate decisions and signal transduction pathways in plants and mammalian systems (Jonak & Hirt, 2002). The non-exhaustive series of proven protein targets of simple triterpenes in mammalian cell lines could be used as a navigation tool in comparative trans-kingdom studies to illustrate the physiological role of saponins in processes pertaining to multicellular organization. On the other hand, knowledge acquired by deciphering the biosynthetic pathways of saponins in plants can provide vital clues to the role of saponins and their specialized intermediates in plant well-being, as well as provide pivotal elements for the rational production of new drugs in synthetic biology approaches. The identification of enzymes responsible for the functionalization of precursors at biologically relevant positions may be exploited for structure-guided compound synthesis in heterologous microbial, plant and animal hosts. Moreover, the identification of regulatory proteins such as transcription factors, protein kinases and phosphatases involved in saponin biosynthesis can be used to augment natural product synthesis in the native plant species. Ancillary to this, systematic studies of the environmental and physiological factors and regulatory proteins that modulate the synthesis of this family of compounds are likely to reveal further as yet unidentified roles for these developmentally and industrially relevant class of compounds.
Acknowledgements
The authors thank James Reed and Jacob Pollier for useful discussions and critically reviewing this document.
Footnotes
Declaration of interest: This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Grant “Understanding and Exploiting Plant and Microbial Secondary Metabolism” (BB/J004596/1), the John Innes Foundation, a joint Engineering and Physical Sciences Research Council/National Science Foundation award to AO as part of the Syntegron consortium (EP/K03459/1), and the EU FP7-funded programme Triterpenes for Commercialization (TriForC; A. O. and K. P.). The authors declare no conflicts of interest.
References
- Abboud HE. Synthetic oleanane triterpenoids: magic bullets or not? Kidney Int. 2013;83:785–7. [PubMed] [Google Scholar]
- Abe I, Ebizuka Y, Sankawa U. Purification of 2,3-oxidosqualene: cycloartenol cyclase from seedlings. Chem Pharm Bull. 1988;36:5031–4. [Google Scholar]
- Amador V, Monte E, García-Martínez JL, Prat S. Gibberellins signal nuclear import of PHOR1, a photoperiod-responsive protein with homology to Drosophila armadillo. Cell. 2001;106:343–54. [PubMed] [Google Scholar]
- Armah CN, Mackie AR, Roy C, et al. The membrane-permeabilizing effect of avenacin A-1 involves the reorganization of bilayer cholesterol. Biophys J. 1999;76:281–90. [PMC free article] [PubMed] [Google Scholar]
- Augustin JM, Kuzina V, Andersen SB, Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry. 2011;72:435–57. [PubMed] [Google Scholar]
- Ayet G, Burbano C, Cuadrado C, et al. Effect of germination, under different environmental conditions, on saponins, phytic acid and tannins in lentils (Lens culinaris) J Sci Food Agric. 1997;74:273–9. [Google Scholar]
- Baisted DJ. Sterol and triterpene synthesis in the developing and germinating pea seed. Biochem J. 1971;124:375–83. [PMC free article] [PubMed] [Google Scholar]
- Barroso-González J, El Jaber-Vazdekis N, García-Expósito L, et al. The lupane-type triterpene 30-oxo-calenduladiol is a CCR5 antagonist with anti-HIV-1 and anti-chemotactic activities. J Biol Chem. 2009;284:16609–20. [PMC free article] [PubMed] [Google Scholar]
- Bednarek P, Osbourn A. Plant-microbe interactions: chemical diversity in plant defense. Science. 2009;324:746–8. [PubMed] [Google Scholar]
- Bejarano L, Mignolet E, Devaux A, et al. Glycoalkaloids in potato tubers: the effect of variety and drought stress on the α-solanine and α-chaconine contents of potatoes. J Sci Food Agric. 2000;80:2096–100. [Google Scholar]
- Boiteau P, Ratsimamanga AR. Asiaticoside extracted from Centella asiatica and its therapeutic uses in cicatrization of experimental and refractory wounds (leprosy, cutaneous tuberculosis and lupus) Therapie. 1956;11:125–49. [PubMed] [Google Scholar]
- Bordbar S, Anwar F, Saari N. High-value components and bioactives from sea cucumbers for functional foods – a review. Mar Drugs. 2011;9:1761–805. [PMC free article] [PubMed] [Google Scholar]
- Bouarab K, Melton R, Peart J, et al. A saponin-detoxifying enzyme mediates suppression of plant defences. Nature. 2002;418:889–92. [PubMed] [Google Scholar]
- Bowyer P, Clarke BR, Lunness P, et al. Host range of a plant pathogenic fungus determined by a saponin detoxifying enzyme. Science. 1995;267:371–4. [PubMed] [Google Scholar]
- Boycheva S, Daviet L, Wolfender JL, Fitzpatrick TB. The rise of operon-like gene clusters in plants. Trends Plant Sci. 2014;19:447–59. [PubMed] [Google Scholar]
- Brendolise C, Yauk YK, Eberhard ED, et al. An unusual plant triterpene synthase with predominant α-amyrin-producing activity identified by characterizing oxidosqualene cyclases from Malus × domestica . FEBS J. 2011;278:2485–99. [PubMed] [Google Scholar]
- Brodersen P, Sakvarelidze-Achard L, Schaller H, et al. Isoprenoid biosynthesis is required for miRNA function and affects membrane association of ARGONAUTE 1 in Arabidopsis . Proc Natl Acad Sci USA. 2012;109:1778–83. [PMC free article] [PubMed] [Google Scholar]
- Buschhaus C, Herz H, Jetter R. Chemical composition of the epicuticular and intracuticular wax layers on adaxial sides of Rosa canina leaves. Ann Bot. 2007;100:1557–64. [PMC free article] [PubMed] [Google Scholar]
- Buschhaus C, Jetter R. Composition differences between epicuticular and intracuticular wax substructures: how do plants seal their epidermal surfaces? J Exp Biol. 2011;62:841–53. [PubMed] [Google Scholar]
- Buschhaus C, Jetter R. Composition and physiological function of the wax layers coating Arabidopsis leaves: β-amyrin negatively affects the intracuticular water barrier. Plant Physiol. 2012;160:1120–9. [PMC free article] [PubMed] [Google Scholar]
- Carelli M, Biazzi E, Panara F, et al. Medicago truncatula CYP716A12 is a multifunctional oxidase involved in the biosynthesis of hemolytic saponins. Plant Cell. 2011;23:3070–81. [PMC free article] [PubMed] [Google Scholar]
- Carter JP, Spink J, Cannon PF, et al. Isolation, characterization, and avenacin sensitivity of a diverse collection of cereal-root-colonizing fungi. Appl Environ Microbiol. 1999;65:3364–72. [PMC free article] [PubMed] [Google Scholar]
- Castillo DA, Kolesnikova MD, Matsuda SP. An effective strategy for exploring unknown metabolic pathways by genome mining. J Am Chem Soc. 2013;135:5885–94. [PubMed] [Google Scholar]
- Cheeke PR. Applications of saponins as feed additives in poultry productioned. Engormix: Poultry Industry; 2009. [Google Scholar]
- Chen RJ, Chung TY, Li FY, et al. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin. 2009;30:61–9. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Guo Q, Liu L, et al. Influence of fertilization and drought stress on the growth and production of secondary metabolites in Prunella vulgaris L. J Med Plant Res. 2011;5:1749–55. [Google Scholar]
- Chen YF, Yang CH, Chang MS, et al. Foam properties and detergent abilities of the saponins from Camellia oleifera . Int J Mol Sci. 2010;11:4417–25. [PMC free article] [PubMed] [Google Scholar]
- Chiang KC, Tsui KH, Chung LC, et al. Celastrol blocks interleukin-6 gene expression via downregulation of NF-κB in prostate carcinoma cells. PLoS One. 2014;9:e93151. [PMC free article] [PubMed] [Google Scholar]
- Chiang LC, Chiang W, Chang MY, et al. Antileukemic activity of selected natural products in Taiwan. Am J Chin Med. 2003;31:37–46. [PubMed] [Google Scholar]
- Chwalek M, Lalun N, Bobichon H, et al. Structure-activity relationships of some hederagenin diglycosides: haemolysis, cytotoxicity and apoptosis induction. Biochim Biophys Acta. 2006;1760:1418–27. [PubMed] [Google Scholar]
- Coates JC. Armadillo repeat proteins: beyond the animal kingdom. Trends Cell Biol. 2003;13:463–71. [PubMed] [Google Scholar]
- Coates JC, Laplaze L, Haseloff J. Armadillo-related proteins promote lateral root development in Arabidopsis . Proc Natl Acad Sci USA. 2006;103:1621–6. [PMC free article] [PubMed] [Google Scholar]
- Confalonieri M, Cammareri M, Biazzi E, et al. Enhanced triterpene saponin biosynthesis and root nodulation in transgenic barrel medic (Medicago truncatula Gaertn.) expressing a novel beta-amyrin synthase (AsOXA1) gene. Plant Biotechnol J. 2009;7:172–82. [PubMed] [Google Scholar]
- Dalsgaard A study of the isolation and characterization of the saponin Quil A. Evaluation of its adjuvant activity, with a special reference to the application in the vaccination of cattle against foot-and-mouth disease. Acta Vet Scand Suppl. 1978;69:7–40. [PubMed] [Google Scholar]
- De Costa F, Yendo AC, Fleck JD, et al. Accumulation of a bioactive triterpene saponin fraction of Quillaja brasiliensis leaves is associated with abiotic and biotic stresses. Plant Physiol Biochem. 2013;66:56–62. [PubMed] [Google Scholar]
- Delis C, Krokida A, Georgiou S, et al. Role of lupeol synthase in Lotus japonicus nodule formation. New Phytol. 2011;189:335–46. [PubMed] [Google Scholar]
- Dinda B, Debnath S, Mohanta BC, Harigaya Y. Naturally occurring triterpenoid saponins. Chem Biodivers. 2010;7:2327–580. [PubMed] [Google Scholar]
- Etebu E. Postharvest pathology and phytochemicals of Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) fruits and wastes. Agric Sci Res. 2012;2:285–94. [Google Scholar]
- Favaloro FGJ, Honda T, Honda Y, et al. Design and synthesis of tricyclic compounds with enone functionalities in rings A and C: a novel class of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 2002;45:4801–5. [PubMed] [Google Scholar]
- Field B, Fiston-Lavier AS, Kemen A, et al. Formation of plant metabolic gene clusters within dynamic chromosomal regions. Proc Natl Acad Sci USA. 2011;108:16116–21. [PMC free article] [PubMed] [Google Scholar]
- Field B, Jordán F, Osbourn A. First encounters – deployment of defence-related natural products by plants. New Phytol. 2006;172:193–207. [PubMed] [Google Scholar]
- Field B, Osbourn AE. Metabolic diversification-independent assembly of operon-like gene clusters in different plants. Science. 2008;320:543–7. [PubMed] [Google Scholar]
- Finlay HJ, Honda T, Gribble GW, et al. Novel A-ring cleaved analogs of oleanolic and ursolic acids which affect growth regulation in NRP.152 prostate cells. Bioorg Med Chem. 1997;7:1769–72. [Google Scholar]
- Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587–605. [PubMed] [Google Scholar]
- Friedman M. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J Agric Food Chem. 2006;54:8655–81. [PubMed] [Google Scholar]
- Friedman M, Roitman JN, Kozukue N. Glycoalkaloid and calystegine contents of eight potato cultivars. J Agric Food Chem. 2003;51:2964–73. [PubMed] [Google Scholar]
- Fujioka S, Yokota T. Biosynthesis and metabolism of brassinosteroids. Annu Rev Plant Biol. 2003;54:137–64. [PubMed] [Google Scholar]
- Fujioka T, Kashiwada Y, Kilkuskie RE, et al. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod. 1994;57:243–7. [PubMed] [Google Scholar]
- Fukuda N, Shan S, Tanaka H, Shoyama Y. New staining methodology: eastern blotting for glycosides in the field of Kampo medicines. J Nat Med. 2006;60:21–7. [Google Scholar]
- Gamas P, Niebel Fde C, Lescure N, Cullimore J. Use of a subtractive hybridization approach to identify new Medicago truncatula genes induced during root nodule development. Mol Plant Microbe Interact. 1996;9:233–42. [PubMed] [Google Scholar]
- Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471–86. [PubMed] [Google Scholar]
- Gauthier C, Legault J, Girard-Lalancette K, et al. Haemolytic activity, cytotoxicity and membrane cell permeabilization of semi-synthetic and natural lupane- and oleanane-type saponins. Bioorg Med Chem. 2009a;17:2002–8. [PubMed] [Google Scholar]
- Gauthier C, Legault J, Lavoie S, et al. Synthesis and cytotoxicity of bidesmosidic betulin and betulinic acid saponins. J Nat Prod. 2009b;72:72–81. [PubMed] [Google Scholar]
- Geisler K, Hughes RK, Sainsbury F, et al. Biochemical analysis of a multifunctional cytochrome P450 (CYP51) enzyme required for synthesis of antimicrobial triterpenes in plants. Proc Natl Acad Sci USA. 2013;110:E3360–7. [PMC free article] [PubMed] [Google Scholar]
- Geuns JMC. Steroid hormones and plant growth and development. Phytochemistry. 1978;17:1–14. [Google Scholar]
- Ginzberg I, Tokuhisa JG, Veilleux RE. Potato steroidal glycoalkaloids: biosynthesis and genetic manipulation. Potato Res. 2009;52:1–15. [Google Scholar]
- Go YS, Lee SB, Kim HJ, et al. Identification of marneral synthase, which is critical for growth and development in Arabidopsis. Plant J. 2012;72:791–804. [PubMed] [Google Scholar]
- Grandmougin-Ferjani A, Dalpé Y, Hartmann MA, et al. Sterol distribution in arbuscular mycorrhizal fungi. Phytochemistry. 1999;50:1027–31. [Google Scholar]
- Guajardo-Flores D, García-Patiño M, Serna-Guerrero D, et al. Characterization and quantification of saponins and flavonoids in sprouts, seed coats and cotyledons of germinated black beans. Food Chem. 2012;134:1312–9. [PubMed] [Google Scholar]
- Güçlü-Ustündağ O, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 2007;47:231–58. [PubMed] [Google Scholar]
- Guhling O, Hobl B, Yeats T, Jetter R. Cloning and characterization of a lupeol synthase involved in the synthesis of epicuticular wax crystals on stem and hypocotyl surfaces of Ricinus communis . Arch Biochem Biophys. 2006;448:60–72. [PubMed] [Google Scholar]
- Guhling O, Kinzler C, Dreyer M, et al. Surface composition of myrmecophilic plants: cuticular wax and glandular trichomes on leaves of Macaranga tanarius . J Chem Ecol. 2005;31:2323–41. [PubMed] [Google Scholar]
- Gus-Mayer S, Brunner H, Schneider-Poetsch HAW, Rüdiger W. Avenacosidase from oat: purification, sequence analysis and biochemical characterization of a new member of the BGA family of β-glucosidases. Plant Mol Biol. 1994;26:909–21. [PubMed] [Google Scholar]
- Haralampidis K, Bryan G, Qi X, et al. A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots. Proc Natl Acad Sci USA. 2001a;98:13431–6. [PMC free article] [PubMed] [Google Scholar]
- Haralampidis K, Trojanowska M, Osbourn AE. Biosynthesis of triterpenoid saponins in plants. Adv Biochem Eng Biotechnol. 2001b;75:31–49. [PubMed] [Google Scholar]
- Hartmann K, Peiter E, Koch K, et al. Chemical composition and ultrastructure of broad bean (Vicia faba L.) nodule endodermis in comparison to the root endodermis. Planta. 2002;215:14–25. [PubMed] [Google Scholar]
- Hayashi H, Hiraoka N, Ikeshiro Y, et al. Seasonal variation of glycyrrhizin and isoliquiritigenin glycosides in the root of Glycyrrhiza glabra L. Biol Pharm Bull. 1998;21:987–9. [PubMed] [Google Scholar]
- Hayashi H, Huang P, Takada S, et al. Differential expression of three oxidosqualene cyclase mRNAs in Glycyrrhiza glabra . Biol Pharm Bull. 2004;27:1086–92. [PubMed] [Google Scholar]
- He JX, Gendron JM, Yang Y, et al. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis . Proc Natl Acad Sci USA. 2002;99:10185–90. [PMC free article] [PubMed] [Google Scholar]
- Helmkamp G, Bonner J. Some relationships of sterols to plant growth. Plant Physiol. 1953;28:428–36. [PMC free article] [PubMed] [Google Scholar]
- Henry M, Rahier A, Taton M. Effect of gypsogenin 3,O-glucuronide pretreatment of Gypsophila paniculata and Saponaria officinalis cell suspension cultures on the activities of microsomal 2,3-oxidosqualene cycloartenol and amyrin cyclases. Phytochemistry. 1992;31:3855–9. [Google Scholar]
- Hernández LE, Cooke DT. Lipid composition of symbiosomes from pea root nodules. Phytochemistry. 1996;42:341–6. [Google Scholar]
- Hoagland RE. Toxicity of tomatine and tomatidine on weeds, crops and phytopathogenetic fungi. Allelopathy J. 2009;23:425–36. [Google Scholar]
- Hoffmann D. Medical herbalism: the science and practice of herbal medicine. Rochester (VT): Inner Traditions, Bear & Co; 2003. [Google Scholar]
- Honda T, Finlaya HJ, Gribble GW, et al. New enone derivatives of oleanolic acid and ursolic acid as inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1997;7:1623–8. [Google Scholar]
- Honda T, Gribble GW, Suh N, et al. Novel synthetic oleanane and ursane triterpenoids with various enone functionalities in ring A as inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 2000a;43:1866–77. [PubMed] [Google Scholar]
- Honda T, Honda Y, Favaloro FGJ, et al. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg Med Chem. 2002;12:1027–30. [PubMed] [Google Scholar]
- Honda T, Rounds BV, Bore L, et al. Novel synthetic oleanane triterpenoids: a series of highly active inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett. 1999;9:3429–34. [PubMed] [Google Scholar]
- Honda T, Rounds BV, Bore L, et al. Synthetic oleanane and ursane triterpenoids with modified rings A and C: a series of highly active inhibitors of nitric oxide production in mouse macrophages. J Med Chem. 2000b;43:4233–46. [PubMed] [Google Scholar]
- Hong DS, Kurzrock R, Supko JG, et al. A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin Cancer Res. 2012;18:3396–406. [PMC free article] [PubMed] [Google Scholar]
- Hostettmann K, Marston A. Saponins (chemistry and pharmacology of natural products). Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- Hostettmann K, Marston A. Saponins, chemistry and pharmacology of natural products. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Huang L, Li J, Ye H, et al. Molecular characterization of the pentacyclic triterpenoid biosynthetic pathway in Catharanthus roseus . Planta. 2012;236:1571–81. [PubMed] [Google Scholar]
- Huhman DV, Berhow MA, Sumner LW. Quantification of saponins in aerial and subterranean tissues of Medicago truncatula . J Agric Food Chem. 2005;53:1914–20. [PubMed] [Google Scholar]
- Itkin M, Heinig U, Tzfadia O, et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science. 2013;341:175–9. [PubMed] [Google Scholar]
- Itkin M, Rogachev I, Alkan N, et al. GLYCOALKALOID METABOLISM1 is required for steroidal alkaloid glycosylation and prevention of phytotoxicity in tomato. Plant Cell. 2011;23:4507–25. [PMC free article] [PubMed] [Google Scholar]
- Ito S, Eto T, Tanaka S, et al. Tomatidine and lycotetraose, hydrolysis products of alpha-tomatine by Fusarium oxysporum tomatinase, suppress induced defense responses in tomato cells. FEBS Lett. 2004;571:31–4. [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Haralampidis K, Papadopoulou K, Osbourn AE. Molecular cloning and characterization of triterpene synthases from Medicago truncatula and Lotus japonicus . Plant Mol Biol. 2003;51:731–43. [PubMed] [Google Scholar]
- Jetter R, Schäffer S, Riederer M. Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ. 2000;23:619–28. [Google Scholar]
- Jonak C, Hirt H. Glycogen synthase kinase 3/SHAGGY-like kinases in plants: an emerging family with novel functions. Trends Plant Sci. 2002;7:457–61. [PubMed] [Google Scholar]
- Kannaiyan R, Hay HS, Rajendran P, et al. Celastrol inhibits proliferation and induces chemosensitization through down-regulation of NF-κB and STAT3 regulated gene products in multiple myeloma cells. Br J Pharmacol. 2011;164:1506–21. [PMC free article] [PubMed] [Google Scholar]
- Kapusta I, Janda B, Stochmal A, Oleszek W. Determination of saponins in aerial parts of barrel medic (Medicago truncatula) by liquid chromatography-electrospray ionization/mass spectrometry. J Agric Food Chem. 2005;53:7654–60. [PubMed] [Google Scholar]
- Kemen AC, Honkanen S, Melton RE, et al. Investigation of triterpene synthesis and regulation in oats reveals a role for β-amyrin in determining root epidermal cell patterning. Proc Natl Acad Sci USA. 2014;111:8679–84. [PMC free article] [PubMed] [Google Scholar]
- Killeen GF, Connolly CR, Walsh GA, et al. The effects of dietary supplementation with Yucca schidigera extract or fractions thereof on nitrogen metabolism and gastrointestinal fermentation processes in the rat. J Sci Food Agric. 1998;76:91–9. [Google Scholar]
- Kim OT, Kim MY, Huh SM, et al. Cloning of a cDNA probably encoding oxidosqualene cyclase associated with asiaticoside biosynthesis from Centella asiatica (L.) Urban. Plant Cell Rep. 2005;24:304–11. [PubMed] [Google Scholar]
- Kim SW, Park SK, Kang SI, et al. Hypocholesterolemic property of Yucca schidigera and Quillaja saponaria extracts in human body. Arch Pharm Res. 2003;26:1042–6. [PubMed] [Google Scholar]
- Kim YJ, Jeon JN, Jang MG, et al. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 2014;38:66–72. [PMC free article] [PubMed] [Google Scholar]
- Kitagawa I. Licorice root. A natural sweetener and an important ingredient in Chinese medicine. Pure Appl Chem. 2002;74:1189–98. [Google Scholar]
- Koziol MJ. Chemical composition and nutritional evaluation of quinoa (Chenopodium quinoa Willd.) J Food Compos Anal. 1992;5:35–68. [Google Scholar]
- Krokida A, Delis C, Geisler K, et al. A metabolic gene cluster in Lotus japonicus discloses novel enzyme functions and products in triterpene biosynthesis. New Phytol. 2013;200:675–90. [PubMed] [Google Scholar]
- Kuljanabhagavad T, Thongphasuk P, Chamulitrat W, Wink M. Triterpene saponins from Chenopodium quinoa Willd. Phytochemistry. 2008;69:1919–26. [PubMed] [Google Scholar]
- Lal H, Zahaf T, Heineman TC. Safety and immunogenicity of an AS01-adjuvanted varicella zoster virus subunit candidate vaccine (HZ/su): a phase-I, open-label study in Japanese adults. Hum Vaccines Immunother. 2013;9:1425–9. [PMC free article] [PubMed] [Google Scholar]
- Lapillonne H, Konopleva M, Tsao T, et al. Activation of peroxisome proliferator-activated receptor gamma by a novel synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 2003;63:5926–39. [PubMed] [Google Scholar]
- Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75:1549–60. [PubMed] [Google Scholar]
- Lee S, Cheng H, King KE, et al. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002;16:646–58. [PMC free article] [PubMed] [Google Scholar]
- Lee ST, Wong PF, Cheah SC, Mustafa MR. Alpha-tomatine induces apoptosis and inhibits nuclear factor-kappa B activation on human prostatic adenocarcinoma PC-3 cells. PLoS One. 2011;6:e18915. [PMC free article] [PubMed] [Google Scholar]
- Leroux-Roels G, Bourguignon P, Willekens J, et al. Immunogenicity and safety of a booster dose of an investigational adjuvanted polyprotein HIV-1 vaccine in healthy adults and effect of administration of chloroquine. Clin Vaccine Immunol. 2014;21:302–11. [PMC free article] [PubMed] [Google Scholar]
- Leshem Y, Levin I. The effect of growing alfalfa on subsequent cotton plant development and on nitrate formation in peat soil. Plant Soil. 1978;50:323–8. [Google Scholar]
- Li F, Goila-Gaur R, Salzwedel K, et al. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc Natl Acad Sci USA. 2003;100:13555–60. [PMC free article] [PubMed] [Google Scholar]
- Li W, Liu Y, Zhang JW, et al. Anti-androgen-independent prostate cancer effects of ginsenoside metabolites in vitro: mechanism and possible structure-activity relationship investigation. Arch Pharm Res. 2009;32:49–57. [PubMed] [Google Scholar]
- Liby K, Honda T, Williams CR, et al. Novel semisynthetic analogues of betulinic acid with diverse cytoprotective, antiproliferative, and proapoptotic activities. Mol Cancer Ther. 2007a;6:2113–19. [PubMed] [Google Scholar]
- Liby KT, Sporn MB. Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev. 2012;64:972–1003. [PMC free article] [PubMed] [Google Scholar]
- Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007b;7:357–69. [PubMed] [Google Scholar]
- Lin JT, Chen SL, Liu SC, Yang DJ. Effect of harvest time on saponins in Yam (Dioscorea pseudojaponica Yamamoto) J Food Drug Anal. 2009;17:116–22. [Google Scholar]
- Liu G, Anderson C, Scaltreto H, et al. QS-21 structure/function studies: effect of acylation on adjuvant activity. Vaccine. 2002;20:2808–15. [PubMed] [Google Scholar]
- Liu HW, Li JK, Zhang DW, et al. Two new steroidal compounds from starfish Asterias amurensis Lutken. J Asian Nat Prod Res. 2008;10:521–9. [PubMed] [Google Scholar]
- Liu Y, Lu WX, Yan MC, et al. Synthesis and tumor cytotoxicity of novel amide derivatives of β-hederin. Molecules. 2010;15:7871–83. [PMC free article] [PubMed] [Google Scholar]
- Livingston AL, Knuckles BE, Teuber LR, et al. Minimizing the saponin content of alfalfa sprouts and leaf protein concentrates. Adv Exp Med Biol. 1984;177:253–68. [PubMed] [Google Scholar]
- Long HS, Stander MA, Van Wyk BE. Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. South Afr J Bot. 2012;82:53–9. [Google Scholar]
- Lorent J, Lins L, Domenech O, et al. Domain formation and permeabilization induced by the saponin α-hederin and its aglycone hederagenin in a cholesterol-containing bilayer. Langmuir. 2014;30:4556–69. [PubMed] [Google Scholar]
- Man S, Gao W, Zhang Y, et al. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia. 2010;81:703–14. [PubMed] [Google Scholar]
- Moco S, Capanoglu E, Tikunov Y, et al. Tissue specialization at the metabolite level is perceived during the development of tomato fruit. J Exp Bot. 2007;58:4131–46. [PubMed] [Google Scholar]
- Montoya J, Solon JA, Cunanan SR, et al. A randomized, controlled dose-finding phase II study of the M72/AS01 candidate tuberculosis vaccine in healthy PPD-positive adults. J Clin Immunol. 2013;33:1360–75. [PMC free article] [PubMed] [Google Scholar]
- Morrissey JP, Osbourn AE. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Rev. 1999;63:708–24. [PMC free article] [PubMed] [Google Scholar]
- Moses T, Pollier J, Thevelein JM, Goossens A. Bioengineering of plant (tri)terpenoids: from metabolic engineering of plants to synthetic biology in vivo and in vitro . New Phytol. 2013;200:27–43. [PubMed] [Google Scholar]
- Mugford ST, Louveau T, Melton R, et al. Modularity of plant metabolic gene clusters: a trio of linked genes that are collectively required for acylation of triterpenes in oat. Plant Cell. 2013;25:1078–92. [PMC free article] [PubMed] [Google Scholar]
- Mweetwa AM, Hunter D, Poe R, et al. Steroidal glycoalkaloids in Solanum chacoense . Phytochemistry. 2012;75:32–40. [PubMed] [Google Scholar]
- Mylona P, Owatworakit A, Papadopoulou K, et al. Sad3 and Sad4 are required for saponin biosynthesis and root development in oat. Plant Cell. 2008;20:201–12. [PMC free article] [PubMed] [Google Scholar]
- Nacif de Abreu I, Mazzafera P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol Biochem. 2005;43:241–8. [PubMed] [Google Scholar]
- Naoumkina MA, Modolo LV, Huhman DV, et al. Genomic and coexpression analyses predict multiple genes involved in triterpene saponin biosynthesis in Medicago truncatula . Plant Cell. 2010;22:850–66. [PMC free article] [PubMed] [Google Scholar]
- Ndamba J, Lemmich E, Mølgaard P. Investigation of the diurnal, ontogenetic and seasonal variation in the molluscicidal saponin content of Phytolacca dodecandra aqueous berry extracts. Phytochemistry. 1994;35:95–9. [PubMed] [Google Scholar]
- Ndungu FM, Mwacharo J, Kimani D, et al. A statistical interaction between circumsporozoite protein-specific T cell and antibody responses and risk of clinical malaria episodes following vaccination with RTSS/AS01E. PLoS One. 2012;7:e52870. [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M, Nojima S, Akiyama T, et al. Interaction of digitonin and its analogs with membrane cholesterol. J Biochem. 1984;96:1231–9. [PubMed] [Google Scholar]
- Nisius A. The stromacentre in Avena plastids: an aggregation of β-glucosidase responsible for the activation of oat-leaf saponins. Planta. 1988;173:474–81. [PubMed] [Google Scholar]
- Nützmann HW, Osbourn A. Gene clustering in plant specialized metabolism. Curr Opin Biotechnol. 2014;26:91–9. [PubMed] [Google Scholar]
- Oda K, Matsuda H, Murakami T, et al. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol Chem. 2000;381:67–74. [PubMed] [Google Scholar]
- Oda K, Matsuda H, Murakami T, et al. Relationship between adjuvant activity and amphipathic structure of soyasaponins. Vaccine. 2003;21:145–51. [PubMed] [Google Scholar]
- Ohana R, Delmer DP, Carlson RW, et al. Identification of a novel triterpenoid saponin from Pisum sativum as a specific inhibitor of the diguanylate cyclase of Acetobacter xylinum . Plant Cell Physiol. 1998;39:144–52. [PubMed] [Google Scholar]
- Ohara S, Ohira T. Plant growth regulation effects of triterpenoid saponins. J Wood Sci. 2003;49:59–64. [Google Scholar]
- Ökmen B, Etalo DW, Joosten MH, et al. Detoxification of α-tomatine by Cladosporium fulvum is required for full virulence on tomato. New Phytol. 2013;198:1203–14. [PubMed] [Google Scholar]
- Oleszek W, Jurzysta M. The allelopathic potential of alfalfa root medicagenic acid glycosides and their fate in soil environments. Plant Soil. 1987;98:67–80. [Google Scholar]
- Oleszek W. Allelopathic potentials of alfalfa (Medicago sativa) saponins: their relation to antifungal and hemolytic activities. J Chem Ecol. 1993;19:1063–74. [PubMed] [Google Scholar]
- Oleszek W. Alfalfa saponins: structure, biological activity, and chemotaxonomy. Adv Exp Med Biol. 1996;405:155–70. [PubMed] [Google Scholar]
- Osbourn AE. Saponins in cereals. Phytochemistry. 2003;62:1–4. [PubMed] [Google Scholar]
- Osbourn A, Goss RJ, Field RA. The saponins: polar isoprenoids with important and diverse biological activities. Nat Prod Rep. 2011;28:1261–8. [PubMed] [Google Scholar]
- Osbourn AE, Clarke BR, Lunness P, et al. An oat species lacking avenacin is susceptible to infection by Gaeumannomyces graminis var. tritici . Physiol Mol Plant Pathol. 1994;45:457–67. [Google Scholar]
- Palmer MA, Bowden BN. Variations in sterol and triterpene contents of developing Sorghum bicolor grains. Phytochemistry. 1977;16:459–63. [Google Scholar]
- Panneerselvam K, Tsukamoto C, Honda N, et al. Saponin polymorphism in the Korean wild soybean (Glycine soja Sieb. and Zucc.) Plant Breed. 2013;132:121–6. [Google Scholar]
- Papadopoulou K, Melton RE, Leggett M, et al. Compromised disease resistance in saponin-deficient plants. Proc Natl Acad Sci USA. 1999;96:12923–8. [PMC free article] [PubMed] [Google Scholar]
- Pareja-Jaime Y, Roncero MIG, Ruiz-Roldán MC. Tomatinase from Fusarium oxysporum f. sp. lycopersici is required for full virulence on tomato plants. Mol Plant Microbe Interact. 2008;21:728–36. [PubMed] [Google Scholar]
- Parente JP, Da Silva BP. Bioactive complex triterpenoid saponins from the Leguminosae family. Nat Prod Commun. 2009;4:143–55. [PubMed] [Google Scholar]
- Park JD, Rhee DK, Lee YH. Biological activities and chemistry of saponins from Panax ginseng C. A. Meyer. Phytochem Rev. 2005;4:159–75. [Google Scholar]
- Patel K, Gadewar M, Tahilyani V, Patel DK. A review on pharmacological and analytical aspects of diosgenin: a concise report. Nat Prod Bioprospect. 2012;2:46–52. [Google Scholar]
- Peng Y, Yang Z, Wang Y, et al. Pathways for the steroidal saponins conversion to diosgenin during acid hydrolysis of Dioscorea zingiberensis C. H. Wright. Chem Eng Res Design. 2011;89:2620–5. [Google Scholar]
- Percival G. Light-induced glycoalkaloid accumulation of potato tubers (Solanum tuberosum L) J Sci Food Agric. 1999;79:1305–10. [Google Scholar]
- Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–36. [PubMed] [Google Scholar]
- Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem Rev. 2010;9:425–74. [PMC free article] [PubMed] [Google Scholar]
- Pollier J, Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–15. [PubMed] [Google Scholar]
- Pollier J, Moses T, González-Guzmán M, et al. The protein quality control system manages plant defence compound synthesis. Nature. 2013;504:148–52. [PubMed] [Google Scholar]
- Popovich DG, Kitts DD. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys. 2002;406:1–8. [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–54. [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72:689–99. [PMC free article] [PubMed] [Google Scholar]
- Qi X, Bakht S, Leggett M, et al. A gene cluster for secondary metabolism in oat: implications for the evolution of metabolic diversity in plants. Proc Natl Acad Sci USA. 2004;101:8233–8. [PMC free article] [PubMed] [Google Scholar]
- Qi X, Bakht S, Qin B, et al. A different function for a member of an ancient and highly conserved cytochrome P450 family: from essential sterols to plant defense. Proc Natl Acad Sci USA. 2006;103:18848–53. [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Ahamed A, Amakawa T, et al. Chromosaponin I specifically interacts with AUX1 protein in regulating the gravitropic response of Arabidopsis roots. Plant Physiol. 2001;125:990–1000. [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Tsurumi S, Amakawa T, et al. Involvement of ethylene and gibberellin signalings in chromosaponin I-induced cell division and cell elongation in the roots of Arabidopsis seedlings. Plant Cell Physiol. 2000;41:1–9. [PubMed] [Google Scholar]
- Rahman A, Tsurumi S. The unique auxin influx modulator chromosaponin I: a physiological overview. Plant Tissue Culture. 2002;12:181–94. [Google Scholar]
- Raju J, Mehta R. Cancer chemopreventive and therapeutic effects of diosgenin, a food saponin. Nutr Cancer. 2009;61:27–35. [PubMed] [Google Scholar]
- Roddick JG, Melchers G. Steroidal glycoalkaloid content of potato, tomato and their somatic hybrids. Theor Appl Genet. 1985;70:655–60. [PubMed] [Google Scholar]
- Saleem M, Murtaza I, Tarapore RS, et al. Lupeol inhibits proliferation of human prostate cancer cells by targeting beta-catenin signaling. Carcinogenesis. 2009;30:808–17. [PMC free article] [PubMed] [Google Scholar]
- San Martín R, Briones R. Industrial uses and sustainable supply of Quillaja saponaria (Rosaceae) saponins. Econ Bot. 1999;53:302–11. [Google Scholar]
- Sandrock RW, Vanetten HD. Fungal sensitivity to and enzymatic degradation of the phytoanticipin alpha-tomatine. Phytopathology. 1998;88:137–43. [PubMed] [Google Scholar]
- Sandrock RW, VanEtten HD. The relevance of tomatinase activity in pathogens of tomato: disruption of the β2-tomatinase gene in Colletotrichum coccodes and Septoria lycopersici and heterologous expression of the Septoria lycopersici β2-tomatinase in Nectria haematococca, a pathogen of tomato fruit. Physiol Mol Plant Pathol. 2001;58:159–71. [Google Scholar]
- Schwarzbach A, Schreiner M, Knorr D. Effect of cultivars and deep freeze storage on saponin content of white asparagus spears (Asparagus officinalis L.) Eur Food Res Technol. 2006;222:32–5. [Google Scholar]
- Seeman P. Transient holes in the erythrocyte membrane during hypotonic hemolysis and stable holes in the membrane after lysis by saponin and lysolecithin. J Cell Biol. 1967;32:55–70. [PMC free article] [PubMed] [Google Scholar]
- Seki H, Ohyama K, Sawai S, et al. Licorice beta-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci USA. 2008;105:14204–9. [PMC free article] [PubMed] [Google Scholar]
- Shabani L, Ehsanpour AA, Asghari G, Emami J. Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ J Plant Physiol. 2009;56:621–6. [Google Scholar]
- Shanmugam MK, Dai X, Kumar AP, et al. Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies. Biochem Pharmacol. 2013;85:1579–87. [PubMed] [Google Scholar]
- Shi J, Arunasalam K, Yeung D, et al. Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food. 2004;7:67–78. [PubMed] [Google Scholar]
- Shibuya M, Katsube Y, Otsuka M, et al. Identification of a product specific beta-amyrin synthase from Arabidopsis thaliana . Plant Physiol Biochem. 2009;47:26–30. [PubMed] [Google Scholar]
- Shibuya M, Zhang H, Endo A, et al. Two branches of the lupeol synthase gene in the molecular evolution of plant oxidosqualene cyclases. Eur J Biochem. 1999;266:302–7. [PubMed] [Google Scholar]
- Simons V, Morrissey JP, Latijnhouwers M, et al. Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae . Antimicrob Agents Chemother. 2006;50:2732–40. [PMC free article] [PubMed] [Google Scholar]
- Sjölander A, Cox JC, Barr IG. ISCOMs: an adjuvant with multiple functions. J Leukocyte Biol. 1998;64:713–23. [PubMed] [Google Scholar]
- Smith PF, Ogundele A, Forrest A, et al. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3′,3′-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob Agents Chemother. 2007;51:3574–81. [PMC free article] [PubMed] [Google Scholar]
- Soltysik S, Wu JY, Recchia J, et al. Structure/function studies of QS-21 adjuvant: assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine. 1995;13:1403–10. [PubMed] [Google Scholar]
- Sousa MC, Varandas R, Santos RC, et al. Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: synergistic effects with miltefosine. PLoS One. 2014;9:e89939. [PMC free article] [PubMed] [Google Scholar]
- Sparg SG, Light ME, Van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94:219–43. [PubMed] [Google Scholar]
- Sporn MB, Liby K, Yore MM, et al. Platforms and networks in triterpenoid pharmacology. Drug Dev Res. 2007;68:174–82. [Google Scholar]
- Suh N, Paul S, Lee HJ, et al. Synthetic triterpenoids, CDDO-imidazolide and CDDO-ethyl amide, induce chondrogenesis. Osteoarthritis Cartilage. 2012;20:446–50. [PubMed] [Google Scholar]
- Sun H, Fang WS, Wang WZ, Hu C. Structure–activity relationships of oleanane- and ursane-type triterpenoids. Bot Stud. 2006a;47:339–68. [Google Scholar]
- Sun H, Yang Z, Ye Y. Structure and biological activity of protopanaxatriol-type saponins from the roots of Panax notoginseng . Int Immunopharmacol. 2006b;6:14–25. [PubMed] [Google Scholar]
- Sun HX, Qin F, Ye YP. Relationship between haemolytic and adjuvant activity and structure of protopanaxadiol-type saponins from the roots of Panax notoginseng . Vaccine. 2005;23:5533–42. [PubMed] [Google Scholar]
- Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–96. [PubMed] [Google Scholar]
- Szakiel A, Pączkowski C, Henry M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem Rev. 2011;10:471–91. [Google Scholar]
- Tamura Y, Miyakoshi M, Yamamoto M. Sakagami H. Alternative medicine. Rijeka, Croatia: InTech; 2012. Application of saponin-containing plants in foods and cosmetics; pp. 85–101. [Google Scholar]
- Tan LL, Cai X, Hu ZH, Ni XL. Localization and dynamic change of saikosaponin in root of Bupleurum chinense . J Integr Plant Biol. 2008;50:951–7. [PubMed] [Google Scholar]
- Teng HM, Fang MF, Cai X, Hu ZH. Localization and dynamic change of saponin in vegetative organs of Polygala tenuifolia . J Integr Plant Biol. 2009;51:529–36. [PubMed] [Google Scholar]
- Thakur M, Melzig MF, Fuchs H, Weng A. Chemistry and pharmacology of saponins: special focus on cytotoxic properties. Bot Targets Ther. 2011;1:19–29. [Google Scholar]
- Thibeault D, Gauthier C, Legault J, et al. Synthesis and structure-activity relationship study of cytotoxic germanicane- and lupane-type 3beta-O-monodesmosidic saponins starting from betulin. Bioorg Med Chem. 2007;15:6144–57. [PubMed] [Google Scholar]
- Thimmappa R, Geisler K, Louveau T, et al. Triterpene biosynthesis in plants. Annu Rev Plant Biol. 2014;65:225–57. [PubMed] [Google Scholar]
- Thomas MT, Kurup R, Johnson AJ, et al. Elite genotypes/chemotypes, with high contents of madecassoside and asiaticoside, from sixty accessions of Centella asiatica of south India and the Andaman Islands: for cultivation and utility in cosmetic and herbal drug applications. Ind Crops Prod. 2010;32:545–50. [Google Scholar]
- Tran K, Risingsong R, Royce DB, et al. The combination of the histone deacetylase inhibitor vorinostat and synthetic triterpenoids reduces tumorigenesis in mouse models of cancer. Carcinogenesis. 2013;34:199–210. [PMC free article] [PubMed] [Google Scholar]
- Tsao T, Kornblau S, Safe S, et al. Role of peroxisome proliferator-activated receptor-gamma and its coactivator DRIP205 in cellular responses to CDDO (RTA-401) in acute myelogenous leukemia. Cancer Res. 2010;70:4949–60. [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto C, Kikuchi A, Harada K, et al. Genetic and chemical polymorphisms of saponins in soybean seed. Phytochemistry. 1993;34:1351–6. [PubMed] [Google Scholar]
- Tsurumi S, Wada S. Chromosaponin I stimulates the elongation of cortical cells in lettuce roots. Plant Cell Physiol. 1995;36:925–9. [Google Scholar]
- Turner EMC. The nature of resistance of oats to the take-all fungus. Distribution of the inhibitor in oat seedlings. J Exp Biol. 1960;11:403–12. [Google Scholar]
- Turner TR, Ramakrishnan K, Walshaw J, et al. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. ISME J. 2013;7:2248–58. [PMC free article] [PubMed] [Google Scholar]
- van der Heijden R, Threlfall DR, Verpoorte R, Whitehead IM. Regulation and enzymology of pentacyclic triterpenoid phytoalexin biosynthesis in cell suspension cultures of Tabernaemontana divaricata . Phytochemistry. 1989;28:2981–8. [Google Scholar]
- Van Maarseveen C, Jetter R. Composition of the epicuticular and intracuticular wax layers on Kalanchoe daigremontiana (Hamet et Perr. de la Bathie) leaves. Phytochemistry. 2009;70:899–906. [PubMed] [Google Scholar]
- Vasishtha H, Srivastava RP. Effect of dehusking and cooking on sapogenols of pigeonpea (Cajanus cajan) genotypes. Indian J Agric Biochem. 2011;24:60–4. [Google Scholar]
- Vasishtha H, Srivastava RP. Genotypic variations in protein, dietary fibre, saponins and lectins in Rajmash beans (Phaseolus vulgaris) Indian J Agric Biochem. 2012;25:150–3. [Google Scholar]
- Vincken JP, Heng L, De Groot A, Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68:275–97. [PubMed] [Google Scholar]
- Voutquenne L, Lavaud C, Massiot G, Le Men-Olivier L. Structure-activity relationships of haemolytic saponins. Pharm Biol. 2002;40:253–62. [Google Scholar]
- Wang Y, Porter WW, Suh N, et al. A synthetic triterpenoid, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO), is a ligand for the peroxisome proliferator-activated receptor gamma. Mol Endocrinol. 2000;14:1550–6. [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Zhu Z, et al. Exploration of the correlation between the structure, hemolytic activity, and cytotoxicity of steroid saponins. Bioorg Med Chem. 2007;15:2528–32. [PubMed] [Google Scholar]
- Wang YY, Zhe H, Zhao R. Preclinical evidences toward the use of triterpenoid CDDO-Me for solid cancer prevention and treatment. Mol Cancer. 2014;13:30. [PMC free article] [PubMed] [Google Scholar]
- Wegel E, Koumproglou R, Shaw P, Osbourn A. Cell type-specific chromatin decondensation of a metabolic gene cluster in oats. Plant Cell. 2009;21:3926–36. [PMC free article] [PubMed] [Google Scholar]
- Wetzel D, Menke W, Dieter R, et al. Escin/diethylammonium salicylate/heparin combination gels for the topical treatment of acute impact injuries: a randomised, double blind, placebo controlled, multicentre study. Br J Sports Med. 2002;36:183–8. [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Gong H. Biological activities and syntheses of steroidal saponins: the shark-repelling pavoninins. Lipids. 2007;42:77–86. [PubMed] [Google Scholar]
- Wu F, Yi Y, Sun P, Zhang D. Synthesis, in vitro inhibitory activity towards COX-2 and haemolytic activity of derivatives of esculentoside A. Bioorg Med Chem Lett. 2007;17:6430–3. [PubMed] [Google Scholar]
- Wu W, Zhang Q, Zhu Y, et al. Comparative metabolic profiling reveals secondary metabolites correlated with soybean salt tolerance. J Agric Food Chem. 2008;56:11132–8. [PubMed] [Google Scholar]
- Xiong J, Kashiwada Y, Chen CH, et al. Conjugates of betulin derivatives with AZT as potent anti-HIV agents. Bioorg Med Chem. 2010;18:6451–69. [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Prasad S, Sung B, et al. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins. 2010;2:2428–66. [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang YC, Chen YF, Chang MH. Foam properties, detergent abilities and long-term preservative efficacy of the saponins from Sapindus mukorossi . J Food Drug Anal. 2010;18:155–60. [Google Scholar]
- Yang CR, Zhang Y, Jacob MR, et al. Antifungal activity of C-27 steroidal saponins. Antimicrob Agents Chemother. 2006;50:1710–4. [PMC free article] [PubMed] [Google Scholar]
- Yendo AC, De Costa F, Gosmann G, Fett-Neto AG. Production of plant bioactive triterpenoid saponins: elicitation strategies and target genes to improve yields. Mol Biotechnol. 2010;46:94–104. [PubMed] [Google Scholar]
- Yokota S, Onohara Y, Shoyama Y. Immunofluorescence and immunoelectron microscopic localization of medicinal substance, Rb1, in several plant parts of Panax ginseng . Curr Drug Discov Technol. 2011;8:51–9. [PubMed] [Google Scholar]
- Yoshiki Y, Kudou S, Okubo K. Relationship between chemical structures and biological activities of triterpenoid saponins from soybean. Biosci Biotechnol Biochem. 1998;62:2291–9. [PubMed] [Google Scholar]
- Zhao X, Zheng L, Si J, et al. Immunocytochemical localization of saikosaponin-d in vegetative organs of Bupleurum scorzonerifolium Willd. Bot Stud. 2013;54:32. [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Liang Z, Han R, Wang X. Impact of fertilization on drought response in the medicinal herb Bupleurum chinense DC.: growth and saikosaponin production. Ind Crops Prod. 2009;29:629–33. [Google Scholar]