- Journal List
- J Clin Invest
- v.76(4); 1985 Oct
- PMC424114

Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system.
Abstract
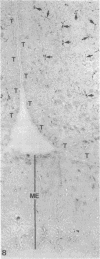
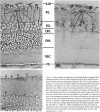
The plasma protein apolipoprotein (apo) E is an important determinant of lipid transport and metabolism in mammals. In the present study, immunocytochemistry has been used to identify apo E in specific cells of the central and peripheral nervous systems of the rat. Light microscopic examination revealed that all astrocytes, including specialized astrocytic cells (Bergmann glia of the cerebellum, tanycytes of the third ventricle, pituicytes of the neurohypophysis, and Müller cells of the retina), possessed significant concentrations of apo E. In all of the major subdivisions of the central nervous system, the perinuclear region of astrocytic cells, as well as their cell processes that end on basement membranes at either the pial surface or along blood vessels, were found to be rich in apo E. Extracellular apo E was present along many of these same surfaces. The impression that apo E is secreted by astrocytic cells was confirmed by electron microscopic immunocytochemical studies, which demonstrated the presence of apo E in the Golgi apparatus. Apo E was not present in neurons, oligodendroglia, microglia, ependymal cells, and choroidal cells. In the peripheral nervous system, apo E was present within the glia surrounding sensory and motor neurons; satellite cells of the dorsal root ganglia and superior cervical sympathetic ganglion as well as the enteric glia of the intestinal ganglia were reactive. Apo E was also present within the non-myelinating Schwann cells but not within the myelinating Schwann cells of peripheral nerves. These results suggest that apo E has an important, previously unsuspected role in the physiology of nervous tissue.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (7.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Mahley RW, Innerarity TL. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. [PubMed] [Google Scholar]
- Mahley RW, Innerarity TL, Rall SC, Jr, Weisgraber KH. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984 Dec 1;25(12):1277–1294. [PubMed] [Google Scholar]
- Hui DY, Innerarity TL, Mahley RW. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- Mahley RW, Hui DY, Innerarity TL, Weisgraber KH. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. [PMC free article] [PubMed] [Google Scholar]
- Angelin B, Raviola CA, Innerarity TL, Mahley RW. Regulation of hepatic lipoprotein receptors in the dog. Rapid regulation of apolipoprotein B,E receptors, but not of apolipoprotein E receptors, by intestinal lipoproteins and bile acids. J Clin Invest. 1983 Apr;71(4):816–831. [PMC free article] [PubMed] [Google Scholar]
- Gordon V, Innerarity TL, Mahley RW. Formation of cholesterol- and apoprotein E-enriched high density lipoproteins in vitro. J Biol Chem. 1983 May 25;258(10):6202–6212. [PubMed] [Google Scholar]
- Hui DY, Harmony JA, Innerarity TL, Mahley RW. Immunoregulatory plasma lipoproteins. Role of apoprotein E and apoprotein B. J Biol Chem. 1980 Dec 25;255(24):11775–11781. [PubMed] [Google Scholar]
- Avila EM, Holdsworth G, Sasaki N, Jackson RL, Harmony JA. Apoprotein E suppresses phytohemagglutinin-activated phospholipid turnover in peripheral blood mononuclear cells. J Biol Chem. 1982 May 25;257(10):5900–5909. [PubMed] [Google Scholar]
- Curtiss LK, DeHeer DH, Edgington TS. In vivo suppression of the primary immune response by a species of low density serum lipoprotein. J Immunol. 1977 Feb;118(2):648–652. [PubMed] [Google Scholar]
- Curtiss LK, Edgington TS. Differential sensitivity of lymphocyte subpopulations to suppression by low density lipoprotein inhibitor, an immunoregulatory human serum low density lipoprotein. J Clin Invest. 1979 Feb;63(2):193–201. [PMC free article] [PubMed] [Google Scholar]
- Wu AL, Windmueller HG. Relative contributions by liver and intestine to individual plasma apolipoproteins in the rat. J Biol Chem. 1979 Aug 10;254(15):7316–7322. [PubMed] [Google Scholar]
- Lin-Lee YC, Tanaka Y, Lin CT, Chan L. Effects of an atherogenic diet on apolipoprotein E biosynthesis in the rat. Biochemistry. 1981 Oct 27;20(22):6474–6480. [PubMed] [Google Scholar]
- Blue ML, Williams DL, Zucker S, Khan SA, Blum CB. Apolipoprotein E synthesis in human kidney, adrenal gland, and liver. Proc Natl Acad Sci U S A. 1983 Jan;80(1):283–287. [PMC free article] [PubMed] [Google Scholar]
- Reue KL, Quon DH, O'Donnell KA, Dizikes GJ, Fareed GC, Lusis AJ. Cloning and regulation of messenger RNA for mouse apolipoprotein E. J Biol Chem. 1984 Feb 25;259(4):2100–2107. [PubMed] [Google Scholar]
- Elshourbagy NA, Liao WS, Mahley RW, Taylor JM. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):203–207. [PMC free article] [PubMed] [Google Scholar]
- Paik YK, Chang DJ, Reardon CA, Davies GE, Mahley RW, Taylor JM. Nucleotide sequence and structure of the human apolipoprotein E gene. Proc Natl Acad Sci U S A. 1985 May;82(10):3445–3449. [PMC free article] [PubMed] [Google Scholar]
- Roheim PS, Carey M, Forte T, Vega GL. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4646–4649. [PMC free article] [PubMed] [Google Scholar]
- Felgenhauer K. Protein size and cerebrospinal fluid composition. Klin Wochenschr. 1974 Dec 15;52(24):1158–1164. [PubMed] [Google Scholar]
- Basu SK, Brown MS, Ho YK, Havel RJ, Goldstein JL. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. [PMC free article] [PubMed] [Google Scholar]
- Basu SK, Ho YK, Brown MS, Bilheimer DW, Anderson RG, Goldstein JL. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Werb Z, Chin JR. Onset of apoprotein E secretion during differentiation of mouse bone marrow-derived mononuclear phagocytes. J Cell Biol. 1983 Oct;97(4):1113–1118. [PMC free article] [PubMed] [Google Scholar]
- Werb Z, Chin JR. Apoprotein E is synthesized and secreted by resident and thioglycollate-elicited macrophages but not by pyran copolymer- or bacillus Calmette-Guerin-activated macrophages. J Exp Med. 1983 Oct 1;158(4):1272–1293. [PMC free article] [PubMed] [Google Scholar]
- Courtoy PJ, Boyles J. Fibronectin in the microvasculature: localization in the pericyte-endothelial interstitium. J Ultrastruct Res. 1983 Jun;83(3):258–273. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. [PMC free article] [PubMed] [Google Scholar]
- Suissa M. Spectrophotometric quantitation of silver grains eluted from autoradiograms. Anal Biochem. 1983 Sep;133(2):511–514. [PubMed] [Google Scholar]
- Mahley RW, Holcombe KS. Alterations of the plasma lipoproteins and apoproteins following cholesterol feeding in the rat. J Lipid Res. 1977 May;18(3):314–324. [PubMed] [Google Scholar]
- Ludwin SK, Kosek JC, Eng LF. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. 1976 Jan 15;165(2):197–207. [PubMed] [Google Scholar]
- Bock E. Nervous system specific proteins. J Neurochem. 1978 Jan;30(1):7–14. [PubMed] [Google Scholar]
- Duffy PE, Graf L, Huang YY, Rapport MM. Glial fibrillary acidic protein in ependymomas and other brain tumors. Distribution, diagnostic criteria, and relation to formation of processes. J Neurol Sci. 1979 Feb;40(2-3):133–146. [PubMed] [Google Scholar]
- Bignami A, Dahl D. Astrocyte-specific protein and neuroglial differentiation. An immunofluorescence study with antibodies to the glial fibrillary acidic protein. J Comp Neurol. 1974 Jan 1;153(1):27–38. [PubMed] [Google Scholar]
- Bignami A, Eng LF, Dahl D, Uyeda CT. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. [PubMed] [Google Scholar]
- WOLTER JR. Glia of the human retina. Am J Ophthalmol. 1959 Nov;48(5):370–393. [PubMed] [Google Scholar]
- Bleier R. The relations of ependyma to neurons and capillaries in the hypothalamus: a Golgi-Cox study. J Comp Neurol. 1971 Aug;142(4):439–463. [PubMed] [Google Scholar]
- Millhouse OE. A Golgi study of third ventricle tanycytes in the adult rodent brain. Z Zellforsch Mikrosk Anat. 1971;121(1):1–13. [PubMed] [Google Scholar]
- Yen SH, Fields KL. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 1981 Jan;88(1):115–126. [PMC free article] [PubMed] [Google Scholar]
- Schachner M, Hedley-Whyte ET, Hsu DW, Schoonmaker G, Bignami A. Ultrastructural localization of glial fibrillary acidic protein in mouse cerebellum by immunoperoxidase labeling. J Cell Biol. 1977 Oct;75(1):67–73. [PMC free article] [PubMed] [Google Scholar]
- Bascó E, Woodhams PL, Hajós F, Balázs R. Immunocytochemical demonstration of glial fibrillary acidic protein in mouse tanycytes. Anat Embryol (Berl) 1981;162(2):217–222. [PubMed] [Google Scholar]
- Suess U, Pliska V. Identification of the pituicytes as astroglial cells by indirect immunofluorescence-staining for the glial fibrillary acidic protein. Brain Res. 1981 Sep 21;221(1):27–33. [PubMed] [Google Scholar]
- Salm AK, Hatton GI, Nilaver G. Immunoreactive glial fibrillary acidic protein in pituicytes of the rat neurohypophysis. Brain Res. 1982 Mar 25;236(2):471–476. [PubMed] [Google Scholar]
- Bignami A, Dahl D. The radial glia of Müller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979 Jan;28(1):63–69. [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980 Aug 14;286(5774):736–737. [PubMed] [Google Scholar]
- Jessen KR, Thorpe R, Mirsky R. Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: an immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. J Neurocytol. 1984 Apr;13(2):187–200. [PubMed] [Google Scholar]
- Barber PC, Lindsay RM. Schwann cells of the olfactory nerves contain glial fibrillary acidic protein and resemble astrocytes. Neuroscience. 1982;7(12):3077–3090. [PubMed] [Google Scholar]
- Dahl D, Chi NH, Miles LE, Nguyen BT, Bignami A. Glial fibrillary acidic (GFA) protein in Schwann cells: fact or artifact? J Histochem Cytochem. 1982 Sep;30(9):912–918. [PubMed] [Google Scholar]
- Bignami A, Dahl D. Specificity of the glial fibrillary acidic protein for astroglia. J Histochem Cytochem. 1977 Jun;25(6):466–469. [PubMed] [Google Scholar]
- Shaw G, Weber K. The structure and development of the rat retina: an immunofluorescence microscopical study using antibodies specific for intermediate filament proteins. Eur J Cell Biol. 1983 May;30(2):219–232. [PubMed] [Google Scholar]
- Deck JH, Eng LF, Bigbee J, Woodcock SM. The role of glial fibrillary acidic protein in the diagnosis of central nervous system tumors. Acta Neuropathol. 1978 Jun 30;42(3):183–190. [PubMed] [Google Scholar]
- Roessmann U, Velasco ME, Sindely SD, Gambetti P. Glial fibrillary acidic protein (GFAP) in ependymal cells during development. An immunocytochemical study. Brain Res. 1980 Oct 27;200(1):13–21. [PubMed] [Google Scholar]
- Fontana A. Astrocytes and lymphocytes: intercellular communication by growth factors. J Neurosci Res. 1982;8(2-3):443–451. [PubMed] [Google Scholar]
- Hirsch MR, Wietzerbin J, Pierres M, Goridis C. Expression of Ia antigens by cultured astrocytes treated with gamma-interferon. Neurosci Lett. 1983 Oct 31;41(1-2):199–204. [PubMed] [Google Scholar]
- Fontana A, Fierz W, Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. [PubMed] [Google Scholar]
- Serougne C, Lefevre C, Chevallier F. Cholesterol transfer between brain and plasma in the rat: a model for the turnover of cerebral cholesterol. Exp Neurol. 1976 Apr;51(1):229–240. [PubMed] [Google Scholar]
Associated Data
Articles from The Journal of Clinical Investigation are provided here courtesy of American Society for Clinical Investigation