- Journal List
- Pharmacogn Rev
- v.7(14); Jul-Dec 2013
- PMC3841988

Natural proteins: Sources, isolation, characterization and applications
Jitendra Y. Nehete
Department of Pharmacognosy, MGV's Pharmacy College, Panchavati, Nashik, India
Rajendra S. Bhambar
Department of Pharmacognosy, MGV's Pharmacy College, Panchavati, Nashik, India
Minal R. Narkhede
1Department of Pharmaceutics, MGV's Pharmacy College, Panchavati, Nashik, India
Sonali R. Gawali
Department of Pharmacognosy, MGV's Pharmacy College, Panchavati, Nashik, India
Abstract
Worldwide, plant protein contributes substantially as a food resource because it contains essential amino acids for meeting human physiological requirements. However, many versatile plant proteins are used as medicinal agents as they are produced by using molecular tools of biotechnology. Proteins can be obtained from plants, animals and microorganism cells. The abundant economical proteins can be obtained from plant seeds. These natural proteins are obtained by isolation procedures depending on the physicochemical properties of proteins. Isolation and purification of single protein from cells containing mixtures of unrelated proteins is achievable due to the physical and chemical attributes of proteins. The following characteristics are unique to each protein: Amino acid composition, sequence, subunit structures, size, shape, net charge, isoelectric point, solubility, heat stability and hydrophobicity. Based on these properties, various methods of isolation exist, like salting out and isoionic precipitation. Purification of proteins is quiet challenging and, therefore, several approaches like sodium dodecyl sulfate gel electrophoresis and chromatography are available. Characterization of proteins can be performed by mass spectrometry/liquid chromatography-mass spectrometry (LC-MS). The amino acid sequence of a protein can be detected by using tandem mass spectrometry. In this article, a review has been made on the sources, isolation, purification and characterization of natural proteins.
INTRODUCTION
For carrying out different body functions, the body regularly needs nutrients like vitamins, minerals, proteins, fiber and carbohydrates, which are obtained from plant or animal sources or both. Among nutrients, the human body requires proteins as the most important compounds because they aid in building cells and tissues and help in repairing the tissues in the body. A high protein diet is recommended for those thinking of building body or muscles. If the body lacks in carbohydrates and fats, the body makes use of proteins for energy production as they are essential for building muscle mass.[1]
The term “complete protein” refers to foods that contain all nine essential amino acids in the correct proportion to build protein in the body. In contrast, “incomplete protein” refers to foods that have all essential amino acids but not in the correct proportion, and are termed as “limiting amino acid.”[2]
Thus, proteins are not only important in the human body but are also widely used in the industry. Hence, an attempt is made to review naturally obtained proteins and its application in pharmaceutical industries.
Building blocks of proteins (Amino acids)
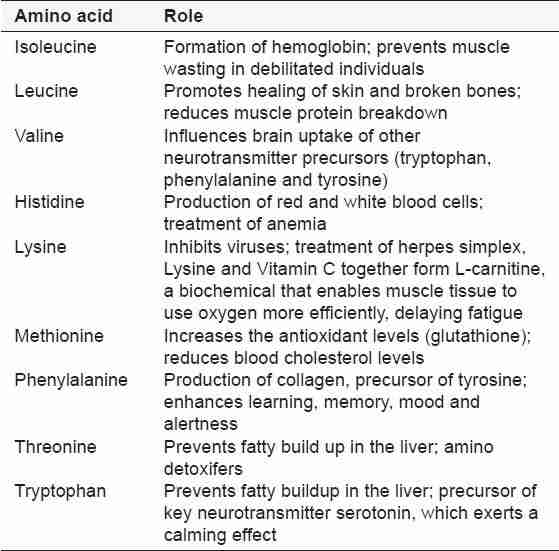
Naturally occurring organic compounds containing amino and carboxyl groups, which are the chief constituents of protein and are necessary for human and animal growth and nutrition, are termed as essential amino acids. Hence, essential amino acid-rich food consumption is an option to source these as they are not produced by the human body.[3] Proteins (or polypeptides) are amino acids joined together by peptide bonds.[4] The roles of the various amino acids are highlighted in Table 1.[5]
Properties of proteins
Solubility
Protein solubility properties are summarized as:
Forms colloidal solutions in water (due to huge size)
Solubility depends on electrostatic charges; net charge depends on number, identity, location of amino acids and pH of solvent
It depends upon isoelectric point (range 5-8.5): Isoelectric point depends on seven-charge amino acids, viz. glutamate (δ-carboxyl group), aspartate (β-carboxyl group), cysteine (thiol group), tyrosine (phenol group), histidine (imidazole side chains), lysine (ε-ammonium group) and arginine (guanidinium group).[5]
Molecular weight
Proteins’ molecular weight variation depends on the number of amino acid residues. Each amino acid contributes 110 value increases in a proteins’ molecular weight, e.g., Insulin - 5700; Myoglobin - 1700; Hemoglobin - 64,450.
Shape
Protein shape varies as globular (insulin), oval (albumin), fibrous or elongated (fibrinogen).
Acidic and basic proteins
Proteins in which the ratio of (is >1, then it is a basic protein and if the value is <1, then it is an acidic protein.
Color reactions of proteins
Useful to identify the nature of the amino acids present in proteins as given in Table 2.[6]
In vivo half-life
This indicates the means time taken by proteins to disappear after its synthesis in cell to its initial half amount, and is predicted using three model organisms (human, yeast and E. coli).[7] “N-end rule,” which relates to the half-life of a protein, is used to identity its N-terminal residue.
Extinction coefficient
It indicates at the amount of light absorbed by a protein at a certain wavelength. This coefficient value helps in estimating and identifying a protein when exposed to a spectrophotometer.[7] The molar extinction coefficient of a protein can be estimated by knowing its amino acid composition. By using the following equation, the native protein extinction coefficient in water can be computed using the molar extinction coefficient values at a given wavelength of tyrosine, tryptophan and cystine (at 280 nm, the extinction value of Tyr is 1490, of Trp is 5500 and of Cys is 125 in water).[8]
E1 = no. of (Tyr) * Ext (Tyr) + no. of (Trp) * Ext (Trp) no. of (Cystine) * Ext (Cystine)
E2 = no. of (Tyr) * Ext (Tyr) + no. of (Trp) * Ext (Trp)
Two values of the proteins produced in water at 280 nm using the above equation indicate that the first value (E1) is due to cysteine residues appearing as half cystines and that the second value (E2) is due to no cysteine appearing as half cystine.
Aliphatic index
The aliphatic index of a protein indicates a relative volume occupied by the aliphatic side chains (alanine, valine, isoleucine and leucine), which also increase the thermostability of the globular proteins with increasing value. This is calculated as below.
Aliphatic index = X (Ala) + a * X (Val) + b * {X (Ile) + X (Leu)}
*a and b coefficients are the relative volumes of the valine side chain (a = 2.9) and of the Leu/Ile side chains (b = 3.9) to the side chain of alanine.[9]
GRAVY (Grand average of hydropathicity)
This is calculated using the Kyte-Doolittle scale[10] as follows:
GRAVY = Sum of AA hydropathy values/residue numbers in sequence
An increasing positive score indicates greater hydrophobicity.
Protein sequencing
The amino acid sequence determination, protein conformation and extent of complexation with any non-peptide molecules in a protein is called as protein sequencing.[11]
Methods of protein sequencing are as follows:
Mass spectrometry
Edman degradation reaction.
Edman degradation reaction
Ordered amino acid composition of a protein can be determined by using this reaction. Peptide sequences up to 50 amino acids long can be sequenced by the Automated Edman sequencer. The reaction scheme with sequencing steps for protein is as follows:
Break any disulfide bridges in protein with an oxidizing agent like performic acid or reducing agent like 2-mercaptoethanol. Re-forming of disulfide bridges is prevented by using a protecting group such as iodoacetic acid. Proteins containing more than one chain are separated and purified
Determine the amino acid composition of each chain
Determine the terminal amino acid of each chain
Break each chain into fragments under 50 amino acids long
Separate and purify fragments
Determine the sequence of each fragment
Repeat with a different pattern of cleavage
Construct a sequence of the overall protein.
Proteins containing 50-70 amino acids cannot be sequenced reliably by the Edman degradation because long protein chains need to be broken up into small fragments that are sequenced individually. Digestion is performed either by endopeptidases such as trypsin or pepsin or by chemical reagents such as cyanogen bromide.
Mass spectrometry
The protein sequence can be directly determined by this technique using electro-spray ionization. A protein of any size can be sequenced by this method, but difficulty arises as the protein size increases. Liquid samples for mass spectrometry can be easily prepared due to greater solubility of peptides as compared with whole proteins. In solution, the proteins are digested by an endoprotease and passed through a high-pressure liquid chromatography column. At the end of this column, the solution is sprayed out of a narrow nozzle charged to a high positive potential into a mass spectrometer. The charge on the droplets causes them to fragment until only single ions remain. Peptides are then fragmented and the mass-to-charge ratio of this is measured. This process is repeated with a different digestion enzyme, and overlaps in sequences are used to construct a sequence for protein.[12]
Determining the amino acid sequences
Amino acid sequence can be determined by hydrolysis, separation, quantitation[13]
Hydrolysis
The peptide chain is hydrolyzed into its amino acid using 6M hydrochloric acid at 110°C for 24 h.
Separation
Separation of amino acids from peptides is performed by eluting the mixture of peptide and buffers (with increasing pH) using a sulfonated polystyrene ion-exchange chromatography column.
Quantitation
Reactions with ninhydrin quantify the amino acids from a peptide in micrograms. This reaction results in an intense blue color for most of the amino acids except proline. Proline gives a yellow color due to the secondary amino group in its structure. The nanogram of an amino acid, determined by fluorescamine, is the component that reacts with the alfa-amino group.
Protein sources: Plant and animal
Animal products, meat, milk, milk products, egg, poultry and fish are rich sources of protein containing a balanced level of amino acids. Plant food items, legumes and nuts are also a source of the same. Animal protein and plant (vegetable) protein are differentiated as:
Animal protein is generally associated with high fat content and, because of this, when consumed in large amounts, it leads to high risks of diseases, including high blood pressure and heart diseases
Animal protein has a balanced combination of all amino acids; hence, it is called complete protein. In contrast, plant (vegetable) protein is incomplete protein; soybean protein is an exception to this.
Plant proteins
Vegetables, legumes and fruits are good sources of protein. Legumes have a higher content of protein than vegetables and fruits.[14] Different parts of plants are sources of proteins as given in Table 3. Plants were studied for isolation of proteins that have applications in the pharmaceutical industry, as shown in Table 4.
Some important proteins, their characteristics and uses are given below.[15]
Soybean proteins
Soy proteins are a mixture of globular proteins - conglycinin (140-170 kDa with glycosylated three subunits) and Glycinin (340-375 kDa with six AB subunits comprised of an acidic [A] and a basic [B] polypeptide linked via disulfide bonds) and are obtained from the plant species Glycine max, family Fabaceae.[35] Based on the molecular weight and sedimentation coefficient, it separates into fractions 2S, 7S, 11S or 15S.[36] The 7S globulin and 11S globulin comprise 37% and 31% of the soy proteins, respectively. In combination with other film-forming proteins, glycinin is known as the gelling agent, emulsifier and foaming agent.[37] B-conglycinin is less heat stable than glycinin and gets denaturated at temperatures of 70°C and 80°C.[38]
Wheat proteins
Based on solubility, the wheat protein fractions are classified as albumins (water soluble), globulins (dilute salt solutions soluble), gliadins (soluble in 70-90% ethanol, comprise 34% of the total protein) and glutenin (insoluble under all of the previously mentioned conditions, comprise 47% of the total protein).[39,40] Gliadin (40 kDa) is a single-chained peptide of four distinct fractions containing intramolecular disulfide bonds.[40] These play a role in film formation, strength and elasticity. Glutenin, a mixture of proteins, has a molecular weight distribution between 100 and 1000 kDa. The disulfide bonds present in glutenin and gliadin help in determining the strength of the protein matrix.[41]
Corn Zein
Zein contains high percentages of non-polar amino acids, namely glutamine (26%), leucine (20%) and proline (10%) and basic and acidic amino acids in low proportions. It is insoluble in water.[42] Two major fractions of zein are α-zein, soluble in 95% ethanol, and β-zein, soluble in 60% ethanol. Commercially, it is used for tablet coating and in biodegradable packaging.[41]
Pea Proteins
It represents a 20-30% fraction extracted from pea seeds, which includes mainly globulins (65-80%) and two minority fractions, albumins and glutelins. Globulins comprise of three different proteins – legumin, vicilin and convicilin.[43] The 11S globulin fraction with a molar mass between 350 and 400 kDa is represented by legumin, while the 7S globulin fraction with a molar mass of 150 kDa is represented by vicilin and convicilin.[44]
Rice Proteins
During processing of white rice, rice bran is obtained, which is a rich source of inexpensive high-quality proteins obtained from grain during the milling process.[45] Compared with rice bran, the protein content in rice grains is slightly lower, varying from 6% to 15%.[46] It is generally prepared by alkali extraction followed by isoelectric precipitation[47,48] and by subcritical water treatment.[49,50] After sequential extraction of the rice protein fractions, the following distribution has been obtained: About 75% glutenin, 15% globulin, 6% albumin and 3% prolamin.[51] The foaming properties of rice protein are similar to those of albumin from egg white; the emulsifying capacities of albumin from bovine serum (BSA) are significantly higher than those of rice proteins; minimum protein solubility is close to isoelectric point at pH 4 and maximum at pH 10; main amino acid content of rice proteins is similar to that of casein and soy proteins; and denaturation temperature of the rice protein isolate is about 83.4°C.[44]
Sunflower Proteins
Proteins are majority constituents in sunflower oil cakes. Defatted sunflower flour contains a high quantity of proteins, around 27% in dry weight.[52] The dehulled seed consists of about 20-40% crude protein. Four fractions of protein are present in the sunflower protein:[53] Globulins, 55-60%; albumins, 17-23% of total proteins; and two minor fractions, glutelins and prolamins, comprising 11-17% and 1-4% of the total protein fractions, respectively. It shows two major fractions: 11S globulins (also named helianthinin) and 2S albumins. Helianthinin has been reported to be present as a globular oligomeric protein with a molecular weight of 300-350 kDa,[54] and this protein mainly exists in the 11S hexametric form.
Animal proteins
Protein from animal sources contains essential amino acids needed for an adult's diet. The important examples are as given below.
Casein
There are four main subunits: s1 α casein (23.6 kDa, 4.94 a pI, net charge - 21.9 at a pH of 6.6), s2 α casein (net charge − 13.8, 5.37 pI, hydrophilic due to high-charge density), β-casein (polar N-terminal amphipathic protein with large hydrophobic domain, Ca 2 + sensitive, at 4°C solubility increases) and κ-casein (not Ca 2 + sensitive), which make up 38%, 10%, 36% and 13% of the casein composition, respectively, and has a unique property to form films.[55,56,57] s1 α casein is amphipathic due to the charge between the hydrophobic N- and C-terminals. It has 8 phosphorylated serine clustered with glutamine residues possessing calcium-binding sites and hence is Ca 2 + sensitive, 17 proline, 25 glutamine residues and no cysteine residues. Here, the Ca 2 + sensitivity means aggregation and precipitation in low ion concentrations. It does not participate in disulfide bond formation and cross-linking due to the absence of free cysteine. Caseins are heat stable because they are proline-rich, which interrupt alfa-helix and beta strands, resulting in the absence of disulfide bridges in the structure. It has relatively little secondary or tertiary structure. These undergo proteolytic cleavage due their open structure imparted due by the high proline content. This characteristic, along with acid-soluble calcium–phosphate bridging, makes an excellent target-activated release mechanism for unloading drug in the stomach.[58,59]
Whey protein
Technically, whey proteins are those that remain in milk serum after coagulating caseins at 4.6 pH and 20°C.[60] It contain β-lactoglobulin (18.3 kDa, containing 160 amino acids),[61] α-lactalbumin (14.2 kDa), BSA (66 kDa, longest single-chain protein) and immunoglobins (thermolabile mixture of proteins).[62,63]
Meat proteins
Sarcoplasmic, stromal and myofibrillar are types of meat protein. Sarcoplasmic proteins contain enzymes myoglobulin and cytoplasmic. Collagen and elastin are the content of stromal proteins while myosin, actin, tropomysin and troponins are the content of myofibrillar proteins. Stromal and myofibrillar proteins, soluble in salt solutions, are used for making edible films and coatings. Collagen, a fibrous stromal protein extracted from connective tissue, tendons, skin, bones and the vascular system, and is a waste products of meat processing. Collagen is a superhelical structure formed by a combination of three parallel alfa-chains, and forms gelatine.[64] Collagen exposed to mild heat treatment under acidic or alkaline conditions forms gelatin.[65]
Egg albumin
Protein fractions: Ovalbumin (44.5 kDa, contains free sulfhydryl groups for cross-linking); ovotransferrin (77.7 kDa, iron-binding protein); ovomucoid, ovomucin and lysozyme, a gram negative antimicrobial present in albumin.[66] Ovalbumin, ovotransferrin and lysozyme protein on denaturation form strong intermolecular beta-sheet structures as heat-set gels due to their thermolabile nature.
Silk proteins
The silkworm Bombyx mori produces silk to weave its cocoon. Biocompatibility, slow bio-degradability, self-assembly, excellent mechanical properties, controllable structure and morphology make it promising materials for drug delivery and tissue engineering.[67] Fibrous protein fibroin, a core of silk and a glue-like protein sericin that envelop fibroin in cocoon formation, are major components of silk.[68]
Isolation of proteins[69]
Selective precipitation of proteins can be used as:
Bulk method to recover majority of the proteins from a crude lysate
Selective method to fractionate a subset of proteins from a protein solution
Specific method to recover a single protein of interest from a purification step.
Selective precipitation methods
Salting out
Isoionic precipitation
Organic co-solvent precipitation
Two carbon (C2) organic co-solvent precipitation of proteins
C4 and C5 organic co-solvent precipitation, phase partitioning and extraction of proteins
Protein exclusion and crowding agents (neutral polymers) and osmolytes
Synthetic and semisynthetic polyelectrolyte precipitation
Metallic and polyphenolic heteropolyanion precipitation
Hydrophobic ion pairing (HIP) entanglement ligands
Matrix-stacking ligand co-precipitation
Di- and trivalent metal cation precipitation.
Salting out
Proteins are salted out as co-precipitate by ammonium sulfate because the saturation concentration provides high molarity that causes precipitation of most proteins. It does not have a large heat of solution and hence the generated heat get easily dissipated; a saturated solution (4.04 M at 20°C) of proteins has a density of 1.235 g/cm3, which does not interfere with the precipitated protein sedimentation by centrifugation. Its concentrated solutions are generally bacteriostatic and protect most proteins from denaturation in solution state.
Figure illustrates the procedures for a pilot experiment.
Dialyze protein samples against a pH buffer or a pH buffer/ammonium sulfate mixture having a sulfate concentration below that needed to start precipitation
Set up a pilot experiment to determine the optimal protein concentration, pH, salt concentration, temperature and incubation time
Add ammonium sulfate to optimal concentration. Incubate (for an optimal period of time) until a precipitate forms
Collect the precipitate by simple decantation
Re-dissolve the precipitate in a buffer suitable for the next step
Perform an assay for the protein of interest.
Isoionic precipitation
Column method
Proteins are frequently least soluble and most precipitable when they are isoionic. In an isoionic, salt-free state, the protein molecules are in their most compact, least-hydrated conformation – a phenomenon that is closely related to the condition of proteins at their isoelectric point. The distinction between isoionic and isoelectric properties is determined by the procedure described by Tanford.[70] Deionization using a column or dialysis aims at rendering proteins isoionic to precipitate them. Two important parameters determine the solubility of many proteins: Solution pH with respect to each proteins’ isoionic point (pI) and low salt concentration (0 to 0.1 to 0.2 M salt). The column method used is appropriate only for proteins that remain soluble at their isoionic point. In addition to adjusting the proteins to their isoionic pH, the general method is using mixed-bed resin deionization to strip away all salts from proteins. Salts, even in small concentration, often have large effects on protein solubility and, therefore, on perceptibility. Inorganic salts tend to salt in many proteins thus enhancing their solubility.
Dialysis method
One of the older methods of rendering proteins salt free or nearly salt free (i.e., isoionic) is dialysis. However, two problems frequently arise with conventional dialysis: (1) When appreciable amounts of protein are present, osmotic effects result in swelling of the dialysis bags as the salt diffuses outward and (2) often, it is quite uncertain where the isoionic point is even if it is feasible to deionize by dialysis against a buffer. The resin deionization method, sometimes called the Dintzis method,[71] automatically adjusts a protein precisely to its isoionic pH without prior knowledge of the same. Salt ions and protein counterions exchange through the membrane and are trapped outside in the exchanger resins. After exchange is complected, the precipitated protein in the dialysis tubing is recovered by centrifugation of the bag's contents. This general technique requires several hours. Salting out and diffusion become slow as the protein concentraction inside the dialysis bag decreases; hence, this method is slower than the flow-through column method.
When proteins are insoluble and precipitate at their isoionic point, deionization is accomplished by placing the dialysis tubing containing the protein sample in a slurry of mixed-bed resin exchangers and incubating with rocking. Proteins insoluble at their isoionic point precipitate inside the bag. The mixed-bed exchange resins remove free salts, forcing the protein to its isoionic pH.
Purification and separation
Sodium dodecyl sulfate (SDS) gel electrophoresis.[72]
This is a technique in which charged molecules such as protein or DNA are separated according to the physical properties as they are forced through a gel by an electrical current.
The principal sample applied to the gel has been treated with detergent SDS and ß-mecaptoethanol. This will denature the proteins and the SDS binding tightly to the uncoiled molecule makes it negatively charged. SDS-polyacrylamide gel electrophoresis (PAGE) gel separates proteins primarily according to size because SDS-coated proteins have a uniform charge: Mass ratio.
One-dimensional gel electrophoresis
This type of electrophoresis can provide information about the molecular size and purity of the proteins as well as the number and molecular size of its subunits. In PAGE, proteins migrate in response to an electrical field through pores in the gel matrix; the pore size decreases with higher acrylamide concentrations. The combination of gel pore size and protein charge, size and shape determines the migration rate of the protein.
Two-dimensional gel electrophoresis
This type of electrophoresis separates proteins in the first dimension by isoelectric focusing and in the second dimension by electrophoresis in the presence of SDS. By separating proteins in this manner, information is obtained not only about the size of a protein, as in one-dimensional gels, but also about the charge of a protein. Two-dimensional gels are superior for resolving complex mixtures and for assessing protein purity.
Application of 2D-PAGE
Applications of 2D-PAGE are summarized as below.
Monitor protein accumulation during development
Comparisons between differentiated organs and tissues
Comparisons of genetic variability within and between species
Detection of protein synthesis effected by various environmental stimuli, such as growth substances (ABA, GA), abiotic stresses (high temperature, low temperature, drought, anaerobiosis and salt) and pathogenic attack.
Separating gel/resolving gel and stacking gel are used in electrophoresis. The composition of the same are: Water, acryl amide mix, tris-HCl buffer (pH 6.8), SDS, ammonium per sulfate and N, N, N\N’- tetramethylethylenediamine.
Protein staining
Coomassie blue staining
This staining requires an acidic medium for generation of an electrostatic attraction between the dye molecules and the amino groups of proteins. Ionic attraction, together with van der Waals forces, binds the dye–protein complex together. Coomassie Blue stains exhibit three-times staining intensity of Fast Green and six-times intensity of Amido Black. Usually, 0.2-0.5 μg of any protein in a sharp band can be detected using Coomassie Blue staining. Because Coomassie Blue is predominantly non-polar, it is usually used in methanolic solution and excess dye is removed from the gel later by distaining. Stacking gels are usually discarded before staining the resolving gels. Gel slabs are placed in staining solution and are stained fully within 4-6 h at room temperature if only 1.5-mm thick.
Silver staining
Silver staining is 50-100-times more sensitive than Coomassie Blue staining. The principal reactive groups are free amines and sulfur groups (basic and sulfur-containing amino acids) contained on proteins. Most proteins stain with monochromatic brown or black colors. Lipoproteins tend to stain blue while some glycoproteins appear yellow, brown or red. Artefact bands with molecular weights ranging from 50 kDa to 68 kDa have been commonly observed in silver-stained gels. Evidence has been presented indicating that these contaminating bands are due to keratin skin proteins.
Characterization
Prior purification is required to characterize proteins, which can be done by a separation mechanism or by chromatographic techniques. Depending on the properties like diameter, electric charge, hydrophobic domains, group and biospecific interactions, a particular technique can be selected. These are summarized in Table 5.
Reversed-phase high-performance liquid chromatography (RP-HPLC)
RP-HPLC, an extremely versatile technique for isolation of synthetically or biologically obtained peptides and proteins is used for both analytical and preparative applications, which involves molecules separation from the mobile phase to immobilized hydrophobic ligands attached to the stationary phase, i.e., sorbent.[73,74,75] The stationary phase containing n-alkyl silica and acetonitrile containing an ionic modifier trifluoroacetic (TFA) are used in RP-HPLC gradient analysis.[74,75] Pharmaceutically important globular proteins, peptides, small polypeptides (molecular weight 10,000) and its related compounds are purified by RP-HPLC. For large-scale protein separation, the use of RP-HPLC is limited because acidic buffering systems and hydrophobicity of n-alkyl silica supports results into low mass yields or loss of biological activity of larger polypeptides.[76,77] In this analysis, 1 μg/μl of the stock sample was prepared in 0.1% (v/v) TFA in water (buffer A) and filtered through a 0.22 μm filter if any undissolved material remained. Baseline was obtained using 100% buffers with a flow rate of 1 mL/min at 215 nm. Ten microliters of the sample was injected using a linear gradient from 0% to 100% buffer B: 100% CH3CN containing 0.1% (v/v) TFA over 30 min to elute the sample. Ten microliters of Milli-Q water was used as the blank. In the literature, the HPLC system with a reversed-phase octadecylsilica (C-18) column (4.6 mm id [internal diameter] ×250 mm length, 5 μm particle size, 300 Å pore size, C18 guard column) had been used.[74]
Mass spectrometry of proteins
Large and polar biomolecules are not easily ionized and transferred into the gas phase; hence, electrospray (ES) and matrix-assisted laser desorption ionization (MALDI) techniques are used. Mass spectrometry in proteomics is used in three major areas.[78]
Recombinant proteins, macromolecule characterization and quality control in the field of biotechnology
Protein identification, either in classical biochemical projects or in large-scale proteomic ones
Detection and characterization of post-translational modifications or any method like any covalent modification that alters mass of a protein using versatility of it to detect the molecular weight of the protein.[79]
Liquid chromatography–mass spectrometry and tandem mass spectrometry of peptides and proteins
Protein and peptide tandem mass spectrometry involves application of two separate stages: LC–MS and LC–MS/MS, to solve problems that require protein identification and software tools to identify proteins by matching mass spectrometric data to protein sequence databases.[80,81] Additionally, LC–MS provides high-quality data for peptide mass as compared with MALDI mass spectrometry in fingerprinting searches. Tessier et al. reported the prediction of plant protein hydrolysate containing small peptide amino acids’ composition performed by LC-MS and capillary electrophoresis-mass spectrometry.[82]
Applications of proteins
Proteins are used in drug and gene delivery systems as protein-based nanocarriers. Extended applications include use in controlled delivery, as a film coater, as hydrogels, as composites, as albumin-based nanoparticles, as microparticles and as beads. Some examples are:
Whey proteins used as hydrogels, nanoparticle systems for encapsulation and controlled delivery of bioactive compounds[83]
As anti-hypertensive use, like genetically modified soybean seeds accumulating novokinin[84]
As solubility enhancer of curcumin in the food industry due to protein–micelle structure (beta-casein), acting as a nano vehicle[85]
As vehicles for bioactives, like milk proteins
As a source of bioactive peptides, e.g., casein-derived four main bioactive peptides act on the cardiovascular system, nervous system, immune system and nutrition system[85]
As a novel antifungal, e.g., Pisumin proteins obtained from Pisum sativum. Sugar snap pea legumes.[86]
In microencapsulation, e.g., vegetable proteins – soy proteins, pea proteins, wheat proteins, rice proteins, oat proteins and sunflower proteins[83]
As pest control: Proteinaceous cysteine proteinase inhibitor, an insecticidal protein found in pulses used to control the proteolytic activity of endogenous digestive cystein proteinase in the mid-gut of some insects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
Articles from Pharmacognosy Reviews are provided here courtesy of Wolters Kluwer -- Medknow Publications