- Journal List
- HHS Author Manuscripts
- PMC2951311

Dietary lignans: physiology and potential for cardiovascular disease risk reduction
Julia Peterson
1Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging and Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA
Johanna Dwyer
1Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging and Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA
2School of Medicine, Tufts University and Frances Stern Nutrition Center, Tufts Medical Center, Boston, MA
Herman Adlercreutz
3Institute for Preventive Medicine, Nutrition, and Cancer, Folkhälsan Research Center, Biomedicum, and Division of Clinical Chemistry, University of Helsinki, Finland
Augustin Scalbert
4Unité de Nutrition Humaine INRA Centre de Recherche de Clermont-Ferrand/Theix 63122 Saint-Genes-Champanelle France
Paul Jacques
1Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging and Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA
Marjorie L McCullough
5Epidemiology Research Program, American Cancer Society, Atlanta, GA
Abstract
We reviewed lignan physiology and lignan intervention and epidemiological studies to determine if they decreased the risks of cardiovascular disease in Western populations. Five intervention studies using flaxseed lignan supplements indicated beneficial associations with C-reactive protein and a meta-analysis, which included these studies, also suggested a lowering effect on plasma total and low-density lipoprotein cholesterol. Three intervention studies using sesamin supplements indicated possible lipid and blood pressure lowering associations. Eleven human observational epidemiological studies examined dietary intakes of lignans in relation to cardiovascular disease risk. Five showed decreased risk with either increasing dietary intakes of lignans or increased levels of serum enterolactone (an enterolignan used as a biomarker of lignan intake), five studies were of borderline significance, and one was null. The associations between lignans and decreased risk of cardiovascular disease are promising, but are yet not well established, perhaps due to low lignan intakes in habitual Western diets. At the higher doses used in intervention studies, associations were more evident.
Introduction
The lignans are bioactive, non-nutrient, non-caloric phenolic plant compounds that are found in highest concentration in flax and sesame seeds and in lower concentrations in grains, other seeds, fruits and vegetables. The enterolignans (sometimes referred to as mammalian lignans) are metabolites of food lignans produced by human intestinal bacteria. They have been identified in human urine and plasma. Their weak estrogenic1 and other biochemical properties suggest potential for nutritional significance in the prevention of cardiovascular and other chronic diseases.2-4 This article briefly describes the chemistry and biosynthesis of lignans in plants (including flaxseed and sesame), major food sources, their metabolism in humans, and recent studies of their associations with cardiovascular disease biomarkers, events and mortality in humans.
Chemistry and occurrence of lignans
Monolignols (Figure 1a), derived from hydroxycinnamic acids (p-coumaric, ferulic, and sinapic acids), are either dimerized to lignans (Figure 1b) in the cell or polymerized into larger lignin structures in the cell wall (Figure 1c). These structurally diverse compounds are involved in plant defense (as antioxidants, biocides, phytoalexins, etc.),5 providing protection against diseases and pests, and possibly participating in plant growth control.6,7
Lignans and lignins are very different and should not be confused with each other. Lignans are stereospecific dimers of these cinnamic alcohols (monolignols) bonded at carbon 8 (C8-C8) (Figure 1b).8
In the plant, lignans (monolignol dimers) usually occur free or bound to sugars.6,7 Diglucosides of pinoresinol, secoisolariciresinol, and syringaresinol are common.9-12 Sesaminol triglucoside and sesaminol diglucoside occur in sesame seeds.13-15 In flax, secoisolariciresinol is present as a diglucoside and is part of an ester-linked complex or oligomer (Figure 2) containing 3-hydroxyl-3-methylglutaric acid, a number of cinnamic acid glycosides (usually ferulic or p-coumaric acid) and the flavonoid herbacetin.16-21
The flavonoid herbacetin can be interchanged with secoisolariciresinol diglucoside. The number of units (n) are usually 1-7 with an average of 3. The terminal unit can have 3-hydroxy-3-methy-glutaric acid, ferulic acid glucoside or p-coumaric acid glucoside. Both cinnamic acid glycosides (ferulic acid glucoside or p-coumaric acid glucoside) are shown here to demonstrate where each one is esterified to secoisolariciresinol diglucoside. Based on work by Strandas et al (2008),18 Struijs et al (2007),19 Struijs et al (2008),20 Struijs et al (2009).21
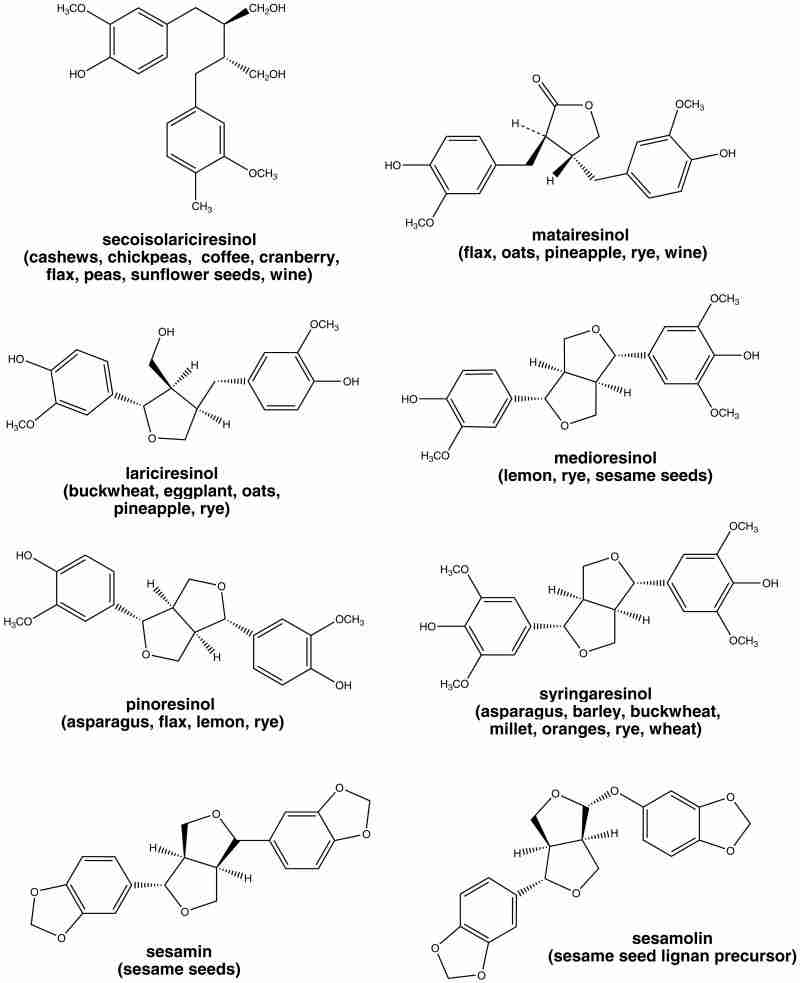
The plant lignans most commonly distributed in foods are lariciresinol, matairesinol, pinoresinol, and secoisolariciresinol (Figure 3). Several other lignans are present in some foods, including medioresinol (in sesame seeds, rye, and lemons), syringaresinol (in grains), sesamin and the lignan precursor sesamolin (in sesame seeds)12,22 (Figure 3). Other lignans found in foods but not often quantified include arctigenin, cyclolariciresinol (isolariciresinol), 7′-hydroxymatairesinol, and 7-hydroxysecoisolariciresinol.2,12 (Some cyclolariciresinol occurs naturally and some is formed from lariciresinol during extraction and analysis under acidic conditions.) The nutritional significance of lignans is unknown. Although lignans are not classified as dietary fibers, they share some of the chemical characteristics of lignin, which is an insoluble fiber.23
Lignins are large plant polymers built from the p-coumaryl, coniferyl, and sinapyl hydroxycinnamic alcohols (see Figure 1c). They are racemic (non-stereospecific) polymers, with monolignol units binding at C8 and four other sites (C5-C5, C5-C8, C5-O-C4, C8-O-C4).24 Lignins are found in vessels and secondary tissues of all higher plants. They are present in a large variety of foods and are particularly abundant in cereal brans.25 Nutritionally lignins are considered components of insoluble dietary fiber.26 Lignins are important in plants because they strengthen the plant cell walls, aid water transport, keep polysaccharides in the plant cell walls from degrading, help plants resist pathogens and other threats, and provide texture in edible plants.24
Food sources of lignans
The lignan content of foods is generally low and usually does not exceed 2 mg/100 g. The exceptions are flaxseed27 (335 mg/100g) and sesame seeds (373 mg/100g),22,28 which have a lignan content a hundred times higher than other dietary sources.
Table 1 provides examples of the distribution of lignans in foods.10,12,28-35 They are present in many plant families, although the types and amounts vary from one family to another. Lignans are found in whole grains (especially in the bran layer) and seeds (in the seed coat). Barley, buckwheat, flax, millet, oats, rye, sesame seeds and wheat contain fairly high levels of lignans. Nuts and legumes are also reasonably good sources. Although in lesser amounts than in grains, lignans are also present in fruits and vegetables such as asparagus, grapes, kiwi fruit, lemons, oranges, pineapple, wine and even in coffee and tea.12,29-32
Table 1
| common food | serving size | Total μg per serving | Total μg per 100 g | Seco | Mat | Lar | Pino | Med | Syr | Family | Genus and species |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beverages | |||||||||||
| coffee, arabica nescafe | 1 tsp | 7 | 694 | 694 | * | Rubiaceae | Coffea arabica | ||||
| coffee, maxwell house | 1 tsp | 5 | 485 | 485 | Rubiaceae | Coffea arabica | |||||
| tea, black china brewed | 8 fl oz | 8 | 3 | 3 | 0 | Theaceae | Camellia sinensis | ||||
| tea, black prince of Wales brewed | 8 fl oz | 19 | 8 | 7 | 1 | Theaceae | Camellia sinensis | ||||
| tea, green china brewed | 8 fl oz | 22 | 9 | 9 | 1 | Theaceae | Camellia sinensis | ||||
| tea, green Japanese sencha brewed | 8 fl oz | 15 | 7 | 6 | 1 | Theaceae | Camellia sinensis | ||||
| wine, cabernet sauvignon, France, red | 5 fl oz | 1117 | 760 | 686 | 74 | Vitaceae | Vitis vinifera | ||||
| wine, chardonnay, France, white | 5 fl oz | 288 | 196 | 174 | 22 | Vitaceae | Vitis vinifera | ||||
| wine, chardonnay, Italy, white | 5 fl oz | 224 | 153 | 136 | 17 | Vitaceae | Vitis vinifera | ||||
| wine, chianti, reserve, Italy, red | 5 fl oz | 2026 | 1378 | 1280 | 98 | Vitaceae | Vitis vinifera | ||||
| Cereals | |||||||||||
| barley whole grain | 1 c | 681 | 370 | 30 | 3 | 85 | 72 | 11 | 169 | Poaceae | Hordeum vulgare |
| barley whole meal | 1 c | 75 | 51 | 51 | 0 | Poaceae | Hordeum spp | ||||
| buckwheat whole grain | 1 c | 1474 | 867 | 131 | 1 | 362 | 92 | 33 | 248 | Polygonaceae | Fagopyrum esculentum |
| corn, whole meal | 1 c | 9 | 7 | 7 | 0 | Poaceae | Zea mays | ||||
| millet, common whole grain | 1 c | 490 | 245 | 67 | 3 | 20 | 85 | 8 | 62 | Poaceae | Panicum miliaceum |
| oat whole grain | 1 c | 1340 | 859 | 19 | 71 | 183 | 194 | 40 | 352 | Poaceae | Avena sativa |
| oat whole meal | 1 c | 19 | 12 | 12 | 0 | Poaceae | Avena sativa | ||||
| rice, brown | 1 c | 26 | 14 | 14 | 0 | Poaceae | Oryza sativa | ||||
| rye whole grain | 1 c | 3196 | 1891 | 38 | 27 | 324 | 381 | 148 | 973 | Poaceae | Secale cereale |
| rye whole meal | 1 c | 128 | 100 | 42 | 58 | Poaceae | Secale cereale | ||||
| wheat whole grain | 1 c | 647 | 539 | 35 | 3 | 62 | 37 | 30 | 372 | Poaceae | Triticum aestivum |
| wheat whole meal (emmer) | 1 c | 9 | 7 | 7 | 0 | Poaceae | Triticum turgidum dicoccoides | ||||
| Fruits | |||||||||||
| apples | 1 med | 0 | 0 | 0 | 0 | Rosaceae | Malus domestica | ||||
| banana | 1 med | 3 | 3 | 3 | 0 | Musaceae | Musa × paradisiaca | ||||
| cantaloupe | 1 med wedge | 12 | 18 | 18 | 0 | Cucurbitaceae | Cucumis melo var cantalupensis | ||||
| cranberry | 1 c | 136 | 136 | 136 | 0 | Ericaceae | Vaccinium macrocarpon | ||||
| currant, black | 1 c | 80 | 72 | 70 | 2 | Grossulariaceae | Ribes nigrum | ||||
| currant, red | 1 c | 30 | 27 | 27 | 0 | Grossulariaceae | Ribes rubrum | ||||
| grapes | 10 grapes | 62 | 126 | 32 | 0 | 37 | 28 | 8 | 21 | Vitaceae | Vitis vinifera |
| guava | 1 fruit | 74 | 134 | 134 | 0 | Myrtaceae | Psidium guajava | ||||
| kiwi | 1 med fruit | 112 | 147 | 116 | 0 | 10 | 8 | 5 | 8 | Actinidiaceae | Actinidia deliciosa |
| lemon | 1 slice | 23 | 335 | 4 | 0 | 25 | 185 | 64 | 57 | Rutaceae | Citrus limon |
| lychee | 1 fruit | 1 | 10 | 10 | 0 | Sapindaceae | Litchi chinensis | ||||
| oranges | 1 fruit | 160 | 122 | 11 | 0 | 19 | 9 | 6 | 77 | Rutaceae | Citrus sinensis |
| papaya | 1 c cubes | 1 | 1 | 1 | 0 | Caricaceae | Carica papaya | ||||
| pineapple | 1 c chunks | 284 | 172 | 7 | 10 | 67 | 4 | 3 | 81 | Bromeliaceae | Ananas comosus |
| plum | 1 fruit | 0 | 1 | 1 | 0 | Rosaceae | Prunus domestica | ||||
| raspberry, red | 10 raspberries | 4 | 20 | 20 | 0 | Rosaceae | Rubus idaeus | ||||
| strawberries | 1 c whole | 206 | 143 | 136 | 7 | Rosaceae | Fragaria × ananassa | ||||
| Nuts, seeds, & spices | |||||||||||
| cashews | 1 oz (18 kernels) | 70 | 247 | 244 | 4 | Anacardiaceae | Anacardium occidentale | ||||
| hazelnut, european hazel | 1 oz (21kernels) | 33 | 116 | 113 | 4 | Betulaceae | Corylus avellana | ||||
| walnuts | 1 oz (14 halves) | 45 | 160 | 156 | 5 | Juglandaceae | Juglans nigra | ||||
| caraway seed | 1 tsp | 4 | 204 | 199 | 5 | Apiaceae | Carum carvi | ||||
| cumin | 1 tsp whole | 4 | 208 | 203 | 5 | Apiaceae | Cuminum cymicum | ||||
| flax seed | 1 tbsp | 34505 | 335002 | 323670 | 5202 | 3670 | 2460 | 0 | 0 | Linaceae | Linum usitatissimum |
| sesame seedˆ | 1 tbsp | 11905 | 132275 | 240 | 1137 | 14835 | 47136 | 4153 | 2050 | Pedaliaceae | Sesamum indicum |
| sunflower seed | 1 oz | 165 | 581 | 581 | 0 | Asteraceae | Helianthus annuus | ||||
| Vegetables & legumes | |||||||||||
| alfalfa | 1 tbsp | 0 | 2 | 2 | 0 | Fabaceae | Medicago spp | ||||
| asparagus | 1 c | 461 | 344 | 183 | 2 | 47 | 49 | 5 | 58 | Asparagaceae | Asparagus officinalis |
| avocado | 1 avocado | 50 | 25 | 21 | 4 | Lauraceae | Persea americana | ||||
| broccoli | 0. 5 c chopped | 21 | 47 | 44 | 2 | Brassicaceae | Brassica oleracea var italica | ||||
| cabbage | 1 c chopped | 2 | 3 | 3 | 0 | Brassicaceae | Brassica oleracea | ||||
| carrot | 1 medium | 14 | 23 | 22 | 0 | Apiaceae | Daucus carota subsp sativus | ||||
| cauliflower | 1 c | 8 | 8 | 8 | 0 | Brassicaceae | Brassica oleracea botrytis | ||||
| celery | 1 c chopped | 5 | 5 | 5 | 0 | Apiaceae | Apium graveolens | ||||
| chickpeas | 1 tbsp | 4383 | 35067 | 35067 | 0 | Fabaceae | Cicer arietinum | ||||
| chives | 1 tbsp | 4 | 117 | 117 | 0 | Liliaceae | Allium schoenoprasum | ||||
| cucumber | 0. 5 c slices | 2 | 4 | 2 | 0 | 1 | 1 | 0 | 0 | Cucurbitaceae | Cucumis sativus |
| eggplant | 1 c cubes | 88 | 107 | 5 | 0 | 68 | 28 | 4 | 2 | Solanaceae | Solanum melongena |
| garlic | 1 clove | 5 | 158 | 157 | 1 | Alliaceae | Allium sativum | ||||
| kidney beans | 1 c | 170 | 92 | 92 | 0 | Fabaceae | Phaseolus vulgaris | ||||
| lentils | 1 tbsp | 0 | 3 | 3 | 0 | Fabaceae | Lens culinaris | ||||
| onion | 1 medium | 11 | 10 | 9 | 1 | Alliaceae | Allium cepa | ||||
| peas | 1 c | 12114 | 8355 | 8355 | 0 | Fabaceae | Pisum sativum | ||||
| peanuts | 1 c halves | 401 | 279 | 279 | 0 | Fabaceae | Arachis hypogaea | ||||
| pepper | 10 strips | 2 | 8 | 7 | 0 | Solanaceae | Capsicum spp | ||||
| potato, sweet | 1 c cubes | 5 | 4 | 2 | 1 | Convolvulaceae | Ipomea batatas | ||||
| radish | 0. 5 c slices | 12 | 21 | 1 | 1 | 14 | 2 | 1 | 2 | Brassicaceae | Raphanus sativus |
| soybeans | 1 c | 243 | 131 | 131 | 0 | Fabaceae | Glycine max | ||||
| tomato | 1 med | 26 | 21 | 1 | 0 | 11 | 5 | 2 | 2 | Solanaceae | Lycopersicon esculentum |
Abbreviations: Seco – secoisolariciresinol, Mat – matairesinol, Lar – lariciresinol, Pino – pinoresinol, Med – medioresinol, Syr – syringaresinol, all as aglycones
In contrast to plants, there are virtually no lignans in animal foods. Minute amounts of the enterolignans enterodiol and enterolactone are sometimes found in animal foods (milk products) as a result of their production by intestinal bacterial metabolism in the animals' guts, but these are exceptions.36-39 Little has been done to investigate the effects of storage and processing on lignans in most foods,29-32,40-45 although it is known that the lignan content is apparently not changed considerably with flaxseed46-50 and sesame seed processing.51-59
Lignan intake
The lignan content of most foods is low and consumption of lignan-rich flaxseed and sesame seed is also low. However, these populations do eat many plant foods that contain small amounts of lignans and they do so often enough to raise their exposure to lignans.60 Lignan intake does not usually exceed 1 mg per day in most Western populations. Estimates of lignan intakes vary from about 150 μg/day61-64 (secoisolariciresinol and matairesinol) to about 1600 μg/day65 (secoisolariciresinol, matairesinol, lariciresinol, pinoresinol, syringaresinol, medioresinol, enterolactone, enterodiol) (Table 2).61-76 Intakes of the two most commonly measured lignans vary from 70 to 1000 μg/day for secoisolariciresinol61,66 and 2 to 74 μg/day for matairesinol.66,67 Methods are now available to quantify lariciresinol and pinoresinol in foods.11,28 Lariciresinol68,69 varies in the diet from 74 to 500 μg/day and pinoresinol67,69 varies from 73 to 423 μg/day. Syringaresinol and medioresinol may also be measured.28
Table 2
| Location | Total | seco | mat | lar | pino | syr | med | enl | end | N | Year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| USA | 106 | 70 | 34 | 545 | 200261 | ||||||
| 579 | 534 | 25 | * | 939 | 200272 | ||||||
| 137 | 110 | 23 | 195 | 200662 | |||||||
| 140 | 115 | 25 | 846b | 200663 | |||||||
| Canada | 857 | 533 | 7 | 74 | 107 | 3471 | 200868 | ||||
| Mexico | 463a | 123 | 2 | 237 | 102 | 50 | 200767 | ||||
| 372a | 123 | 2 | 174 | 73 | 50 | 200767 | |||||
| Finland | 434a | 396 | 38 | 2862b,e | 200370 | ||||||
| France | 1112 | 178 | 11 | 500 | 423 | 58049 | 200769 | ||||
| Germany | 563 | 529 | 29 | 666 | 200473 | ||||||
| 183 | 167 | 15 | 7 | 200574 | |||||||
| 570 | 549 | 21 | 47 | 200574 | |||||||
| Italy | 666 | 335 | 21 | 176 | 97 | 242d | 200975 | ||||
| Netherlands | 1081 | 992 | 74 | 16165 | 200566 | ||||||
| 977 | 152 | 11 | 476 | 334 | 570c | 200676 | |||||
| 979 | 191 | 9 | 488 | 362 | 637b | 200771 | |||||
| Sweden | 1632 | 115 | 24 | 179 | 149 | 831 | 322 | 9 | 0 | 45448 | 200865 |
| UK | 110 | 101 | 8 | 108e | 200564 | ||||||
| 149 | 142 | 9 | 108 | 200564 |
Notes:
Abbreviations: seco - secoisolariciresinol, mat - matairesinol, lar - lariciresinol, pino - pinoresinol, syr - syringaresinol, med - medioresinol, enl - enterolactone, end - enterodiol
NB: Studies that provided only combined values for matairesinol and secoisolariciresinol were not included.
Total lignan intakes vary from country to country because of different dietary sources, but they vary even more depending on variations in the completeness of the food composition tables used, other methodological differences, and on how many individual lignans were analyzed and reported by investigators. More recent studies tend to have more complete analyses.60 Valsta et al's 2003 study70 measuring only matairesinol and secoisolariciresinol found the mean total lignan intake of Finns to be 434 μg/day. Milder et al's 2005 study71 of lariciresinol, matairesinol, pinoresinol, and secoisolariciresinol found median total lignan intake of the Dutch was 979 μg/day. Hedelin et al's 2008 study65 including lariciresinol, matairesinol, medioresinol, pinoresinol, secoisolariciresinol and syringaresinol of Swedish women had a median total lignan intake of 1632 μg/day. These studies indicate that, as expected, when more lignans are measured and quantified in foods, total lignan intakes increase. This challenges the interpretation of studies, particularly of meta-analyses, on lignans and health because it is difficult to compare the intakes that were reported. Muir et al17 and Li et al16 discuss these issues in greater depth using examples from their work on secoisolariciresinol in flaxseed, its diglucoside and its oligomer.
Lignan metabolism
Absorption of plant lignans and bioconversion of plant lignans to enterolignans and their subsequent absorption varies greatly from person to person. Lignans are present in plants both as aglycones (without sugars) and as glycosides (with sugars).12 At present, only in flaxseed has secoisolariciresinol been found as a lignan oligomer. Lignan glycosides are absorbed in the gastrointestinal tract after metabolism by intestinal bacteria to lignan aglycones and the enterolignans (enterolactone and enterodiol), which are formed from them. The extent of hydrolysis to release the lignans from the sugars (and in flax from the oligomer), the formation of enterolignans, and the bioavailability of these compounds vary quite significantly from person to person. Due to these differences in metabolism in the gastrointestinal tract, lignan intake is an imperfect measure of tissue exposure.77,78
Bacterial metabolism in the gut
Lignan glycosides, such as the flax secoisolariciresinol diglucoside ester-linked complex17,79 and the sesame seed sesamolin triglucoside,14,22,80 are hydrolyzed by some of the anaerobic microbes in the gut to lignan aglycones.80-83 The free lignans are then converted into enterolignans through a series of metabolic reactions by various gut bacteria77,84-86 (Figure 4). The efficiency of conversion depends on many factors, and differs considerably from one individual to another. The metabolism of the lignans in the tissues is influenced by genetic factors, but as yet these are not well understood.87-90
The predominant plant lignan compound in foods, secoisolariciresinol diglucoside, is metabolized in the gut to secoisolariciresinol, then to the enterolignan enterodiol and finally to enterolactone, but the conversion is never 100%. The plant lignan matairesinol is metabolized directly in the gut to the enterolignan enterolactone. In an in-vitro fecal microflora metabolism system, lariciresinol was completely converted in 24 hours into the enterolignans enterolactone (46%) and enterodiol (54%) whereas other plant lignans were incompletely converted – matairesinol (62%), secoisolariciresinol diglucoside (72%), and pinoresinol diglucoside (55%). All four were metabolized to enterolactone in part, but secoisolariciresinol and pinoresinol diglucosides were converted to enterodiol (50% of the secoisolariciresinol and 32% of the pinoresinol total doses) and then in small amounts to enterolactone (21% of the secoisolariciresinol and 19% of the pinoresinol total doses).9 Other lignans that are metabolized to enterolactone include arctigenin, 7-hydroxymatairesinol, sesamin, and syringaresinol.9,22,77 Smeds et al91 found cyclolariciresinol, lariciresinol and matairesinol but not secoisolariciresinol in serum samples from a Finnish population. Penalvo et al22,92 determined the presence of cyclolariciresinol, lariciresinol, matairesinol, pinoresinol, as well as anhydrosecoisolariciresinol, 7′-hydroxymatairesinol, secoisolariciresinol, and sesamin in plasma of Finns after the ingestion of sesame seeds (50 g).
The enterolignans enterodiol and enterolactone have been detected in the blood and urine of both humans and animals, but only small amounts of the plant lignans cyclolariciresinol, lariciresinol, matairesinol, pinoresinol, secoisolariciresinol, and syringaresinol have been found in human urine.6,93 In contrast, lignins are thought to be largely inert and not absorbed in the human gut due to their polymeric nature.94-97 It is possible that they are dietary precursors of enterolignans, but the ability of gut bacteria to transform and metabolize lignins into enterolignans has yet to be demonstrated in human studies.25 This possibility is worth pursuing since conversion of food lignins to lignans might explain the relatively high concentrations of enterolignans in biofluids compared to lignan intakes.98
Enterolactone is the main circulating enterolignan and therefore serum enterolactone levels and urinary enterolactone excretion are used as biomarkers for plant lignan intakes. However, these are imperfect surrogates. Differences between lignan intakes and enterolactone production may arise because of variations in the composition of the gut microflora, conversion of some lignans into other compounds, intestinal transit time, the metabolic half-life of enterolactone, the redox state of the colon, the types of lignans present in the diet, and the use of antibiotics.77,84,99
Systemic metabolism
Once they are formed from the parent plant lignans by gut microbiota, the enterolignans enterodiol and enterolactone are absorbed through the colonic barrier,100 and most are conjugated to glucuronides in the tissues. They are usually detectable in the blood 8 to 10 hours after dietary intake.77,78 In a recent study some plant lignans (anhydrosecoisolariciresinol, 7′-hydroxymatairesinol, cyclolariciresinol, lariciresinol, matairesinol, pinoresinol, secoisolariciresinol, and sesamin) were rapidly absorbed in the small intestine and appeared in the systemic circulation within an hour after the ingestion of sesame seeds.22 The mechanisms responsible for the uptake of plant lignans in the small intestine are still unknown.77,85 The pharmacokinetic characterization of lignans is an under-researched area that must be pursued if further insights are to be gained about the actual lignan compounds providing putative health benefits.
The enterolignans either enter enterohepatic circulation or are excreted in the urine, usually as glucuronides and sulfate esters.100-102 Some free lignans and aliphatic or aromatic hydroxylated metabolites from hepatic metabolism may also be excreted.77,85,102-104 One study found that the total amount of enterolactone and enterodiol detected in the urine was up to 40% of the ingested dose (0.9 mg/kg body wt, average 60-66 mg) of secoisolariciresinol diglucoside, and the majority of it was excreted within two days.78
The enterohepatic recirculation of secoisolariciresinol, sesame lignans and enterolignans is significant. In general, lignans permeating the gastrointestinal mucosa are likely to undergo extensive first pass metabolism by phase II enzymes, resulting in glucuronidation or sulfation, either in the mucosa and/or in the liver prior to their appearance in the systemic circulation.100 Glucuronides and sulfates of secoisolariciresinol, enterolactone and enterodiol may undergo enterohepatic recirculation or simply be eliminated in the bile or urine.86,105-108
Lignan intakes, as evaluated with available food composition data and dietary records or even with biomarkers, are such imperfect estimates of exposure that they may obscure diet-disease relationships. In Horn-Ross et al's lignan food frequency questionnaire validation study62 using only matairesinol and secoisolariciresinol, the correlations with urinary total enterodiol and enterolactone were only 0.16. In Bhakta et al's food frequency questionnaire validation study64 the correlation of matairesinol and secoisolariciresinol “true intake” with plasma enterolactone was only 0.11. Since several other lignans are present in the diet and can be converted to enterolactone or enterodiol at varying rates, and some lignans are absorbed without conversion, such low correlations are not surprising. However, these problems do point to the need to improve dietary assessment methodology for these compounds.
Animal and cell lignan studies in cardiovascular areas
There are some animal109-119 and a few in vitro cell120-122 studies on food lignans in the area of cardiovascular disease. For purposes of this review, we have limited the focus to humans and only to food lignans, but the area is worth investigating further. However, caution is indicated since rodent, particularly rat, diets contain other phytoestrogens that may influence results. Several non-food lignans,123,124 such as honokiol125, 126 and magnolol,127-130 have shown cardiovascular associations in animal and in vitro cell studies.
Lignans and cardiovascular disease risk factors
Randomized controlled trials
Most of the controlled trials to date have not used standardized, well-characterized products in which the lignans and other bioactive constituents are quantified. Dose-response data are often incomplete; so the appropriate lignan dose to obtain beneficial health effects is unknown. Another limitation of the epidemiological studies is that, in Western diets, usual lignan intakes are extremely low. It is possible, given the positive results in some interventional studies with higher levels of lignan intakes, that usual intakes are below the threshold necessary to produce cardiovascular effects. Interventional studies with higher doses may provide more insight into the associations of lignans with cardiovascular disease. In addition, other components, such as unsaturated fatty acids present in intact flaxseed and sesame seed, could influence cardiovascular disease risk factors.
There are currently eight randomized controlled trials of lignan supplementation and blood pressure or other intermediate markers of cardiovascular disease risk in the literature; five using secoisolariciresinol diglucoside from flaxseed and three using sesamin from sesame seed. In addition, there is a recently published comprehensive meta-analysis131 of the associations of flaxseed interventions, which included the five studies of flaxseed lignans on cardiovascular disease risk.
The population assessed may have a significant bearing on the outcomes of the lignan intervention. It is important to note that some studies were of healthy volunteers, which may show few associations with risk factors, while others were of individuals at risk.
Blood pressure studies
As shown in Table 3,122,132-134 in a randomized double blind placebo-controlled trial of lignan supplementation in ninety-two healthy older individuals with a walking program, a daily dose of 543 mg secoisolariciresinol diglucoside (187 mg secoisolariciresinol) plus exercise for six months significantly reduced diastolic blood pressure (-2 mm Hg) in middle-aged hypertensive Canadian men (n=42) but did not in women (n=50), and had no association with systolic blood pressure.132 In a Chinese study of seventy-three type 2 diabetics of the same age range who were fed 360 mg secoisolariciresinol diglucoside (124 mg secoisolariciresinol) per day for twelve weeks no significant associations with systolic or diastolic blood pressure were observed at this dose.133
Table 3
| Outcome | Author, year, reference | Adult population | Vehicle | Lignan dosage per day | Study duration weeks | N | Response |
|---|---|---|---|---|---|---|---|
| Systolic | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.9 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 73 | -0.4 mmHg p = 0.268 |
| Cornish et al (2009)132 | M Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 42 | No effect | |
| Cornish et al (2009)132 | F Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 50 | No effect | |
| Wu et al (2009)122 | M, F, Australia, overweight (BMI 25-35), ≥ risk factor metabolic syndrome* or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | -0.7 mmHg p = 0.835 | |
| Miyawaki et al (2009)134 | M, F, Japan, middle aged, mild hypertensives | Capsule 180 g wheat germ oil | Sesamin 60 mg | 4 | 25 | -3.5 mmHg p =0.044 | |
| Diastolic | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 73 | -1.5 mmHg p = 0.751 |
| Cornish et al (2009)132 | M Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 42 | -2 mmHg p = 0.046 | |
| Cornish et al (2009)132 | F Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 50 | No effect | |
| Wu et al (2009)122 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | 0.3 mmHg p = 0.223 | |
| Miyawaki et al (2009)134 | M, F, Japan, middle aged, mild hypertensives | Capsule 180 g wheat germ oil | Sesamin 60 mg | 4 | 25 | -1.9 mmHg p =0.045 | |
| 20-HETE, plasma | Wu et al (2009)122 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | -236 pmol/mmol p = 0.001 |
| 20-HETE, urine / creatinine | Wu et al (2009)122 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | -47 pmol/L p = 0.001 |
Abbreviations: M – males, F – females, BMI – body mass index, BP – blood pressure, LDL – low density lipoprotein cholesterol, Seco – Secoisolariciresinol, SDG - Secoisolariciresinol diglucoside
However, in a Japanese double blind crossover placebo controlled study, 60 mg sesamin (in 180 mg wheat germ oil) per day for four weeks significantly reduced both diastolic (-1.9 mm Hg) and systolic (-3.9 mm Hg) blood pressure in mildly hypertensive middle-aged men (n=23) and women (n=2).134 But among thirty-three overweight Australian men and women with one or more risk factors for metabolic syndrome, fed ∼50 mg per day of sesame lignans in a five week randomized controlled crossover study to determine if the sesame supplement reduced 20-hydroxyeicosatetraenoic acid (20-HETE) (a metabolite of arachidonic acid and a proposed prohypertensive agent in humans), neither systolic nor diastolic blood pressure were lowered. However, the sesame lignan supplementation decreased both plasma and urine 20-HETE significantly, suggesting that lignans may have other cardiovascular disease risk modulating activity.122
Lipoprotein studies
As shown in Table 4,131-133, 135-139 in the Chinese study of seventy-three diabetics who were fed 360 mg secoisolariciresinol diglucoside (124 mg secoisolariciresinol) per day for twelve weeks, no associations with lipid profiles, fasting glucose levels or vascular sensitivity were evident although glycemic control was improved.133 When twenty-two healthy Danish postmenopausal women were fed 500 mg secoisolariciresinol diglucoside (172 mg of secoisolariciresinol) per day for six weeks, secoisolariciresinol did not have any significant association with total cholesterol, high density lipoprotein cholesterol (HDL), low density lipoprotein cholesterol (LDL) or triglycerides.135
Table 4
| Outcome | Author, year, reference | Adult population | Vehicle | Lignan dosage per day | Study duration weeks | N | Response |
|---|---|---|---|---|---|---|---|
| HDL | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 73 | -0.77 mg/dl p = 0.243 |
| Zhang et al (2008)136 | M, W, China, LDL ≥ 3.62 mmol/L hypercholesterolemic | Tablets flaxseed lignan complex | Seco 103 mg (300 mg SDG) | 8 | 18 | -3.86 mg/dl p < 0.001 from baseline | |
| Hallund et al (2006)135 | F, Denmark, postmenopausal age 61 ± 7, healthy, BP<160/90 | Muffin with flaxseed lignan complex | Seco 172 mg (500 mg SDG) | 6 | 22 | -1.54 mg/dl p = 0.531 | |
| Zhang et al (2008)136 | M, W, China, LDL ≥ 3.62 mmol/L hypercholesterolemic | Tablets flaxseed lignan complex | Seco 206 mg (600 mg SDG) | 8 | 55 | -5.4 mg/dl p = 0.167 | |
| Wu et al (2009b)139 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome* or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | 0.00 mg/dl p = 0.764 | |
| Hirata et al (1996)138 | M, Japan, hypercholesterolemic | Capsule 180 g wheat germ oil | Sesamin 32.4 mg 4 weeks then 64.8 mg 4 weeks | 8 | 12 | No effect | |
| LDL | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 73 | -4.25 mg/dl p = 0.404 |
| Zhang et al (2008)136 | M, W, China, LDL ≥ 3.62 mmol/L hypercholesterolemic | Tablets flaxseed lignan complex | Seco 103 mg (300 mg SDG) | 8 | 18 | -28.6 mg/dl p < 0.001 from baseline | |
| Hallund et al (2006)135 | F, Denmark, postmenopausal age 61 ± 7, healthy, BP<160/90 | Muffin with flaxseed lignan complex | Seco 172 mg (500 mg SDG) | 6 | 22 | -7.72 mg/dl p = 0.184 | |
| Cornish et al (2009)132 | M, F Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 92 | No effect | |
| Zhang et al (2008)136 | M, W, China, LDL ≥ 3.62 mmol/L hypercholesterolemic | Tablets flaxseed lignan complex | Seco 206 mg (600 mg SDG) | 8 | 55 | -38.6 mg/dl p = 0.003 | |
| Marblestone 2008137 | F, USA, perimenopausal, age 36-48 | Flaxseed source by Brevail | Seco 69 mg (200 mg SDG) | 14 | 11 | reduced | |
| Pan et al (2009)131 | Meta-analysis, 7 comparisons | Flaxseed lignan supplements | -6.18 mg/dl p = 0.03 | ||||
| Wu et al (2009b)139 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | 2.70 mg/dl p = 0.292 | |
| Hirata et al (1996)138 | M, Japan, hypercholesterolemic | Capsule 180 g wheat germ oil | Sesamin 32.4 mg 4 weeks then 64.8 mg 4 weeks | 8 | 12 | -30.7 mg/dl p <0.05 | |
| Total cholesterol | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 73 | -6.56 mg/dl p = 0.367 |
| Zhang et al (2008)136 | M, W, China, LDL ≥ 3.62 mmol/L hypercholesterolemic | Tablets flaxseed lignan complex | Seco 103 mg (300 mg SDG) | 8 | 18 | -39.8 mg/dl p <0.001 from baseline | |
| Hallund et al (2006)135 | F, Denmark, postmenopausal age 61 ± 7, healthy, BP<160/90 | Muffin with flaxseed lignan complex | Seco 172 mg (500 mg SDG) | 6 | 22 | -8.88 mg/dl p = 0.262 | |
| Cornish et al (2009)132 | M, F Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 92 | No effect | |
| Zhang et al (2008)136 | M, W, China, LDL ≥ 3.62 mmol/L hypercholesterolemic | Tablets flaxseed lignan complex | Seco 206 mg (600 mg SDG) | 8 | 55 | -68.3 mg/dl p <0.001 | |
| Pan et al (2009)131 | Meta-analysis, 7 comparisons | Flaxseed lignan supplements | -10.81 mg/dl p = 0.04 | ||||
| Wu et al (2009b)139 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | 0.77 mg/dl p = 0.227 | |
| Hirata et al (1996)138 | M, Japan, hypercholesterolemic | Capsule 180 g wheat germ oil | Sesamin 32.4 mg 4 weeks then 64.8 mg 4 weeks | 8 | 12 | -23.7 mg/dl p <0.05 | |
| Triglycerides | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan complex | Seco 124 mg (360 mg SDG) | 12 | 73 | -17.70 mg/dl p = 0.720 |
| Zhang et al (2008)136 | M, W, China, LDL ≥ 3.62 mmol/L hypercholesterolemic | Tablets flaxseed lignan complex | Seco 103 mg (300 mg SDG) | 8 | 18 | -46.0 mg/dl not significant from baseline | |
| Hallund et al (2006)135 | F, Denmark, postmenopausal age 61 ± 7, healthy, BP<160/90 | Muffin with flaxseed lignan complex | Seco 172 mg (500 mg SDG) | 6 | 22 | 3.5 mg/dl p = 0.595 | |
| Cornish et al (2009)132 | M Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 49 | Group effect (controls increased p = 0.017) | |
| Cornish et al (2009)132 | F Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 52 | No effect | |
| Zhang et al (2008)136 | M, W, China, LDL ≥ 3.62 mmol/L hypercholesterolemic | Tablets flaxseed lignan complex | Seco 206 mg (600 mg SDG) | 8 | 55 | -76.1 mg/dl p = 0.068 | |
| Wu et al (2009b)139 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | -10.6 mg/dl p = 0.254 | |
| Hirata et al (1996)138 | M, Japan, hypercholesterolemic | Capsule 180 g wheat germ oil | Sesamin 32.4 mg 4 weeks then 64.8 mg 4 weeks | 8 | 12 | No effect | |
| Apo A1 | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 73 | -2.5 mg/dl p = 0.751 |
| Apo B | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 73 | -1.6 mg/dl p = 0.528 |
| Hirata et al (1996)138 | M, Japan, hypercholesterolemic | Capsule 180 g wheat germ oil | Sesamin 64.8 mg | 8 | 12 | -20.3 mg/dl p <0.05 | |
| Lp(a) | Pan et al (2007)133 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 62 | -2.52 mg/dl p = 0.339 |
| Marblestone 2008137 | F, USA, perimenopausal, age 36-48 | Flaxseed source by Brevail | Seco 69 mg (200 mg SDG) | 14 | 11 | reduced |
Abbreviations: Apo A1 – Apolipoprotein A1, Apo B – Apolipoprotein B, Lp(a) – Lipoprotein (a), M– males, F – females, BMI – body mass index, BP – blood pressure, LDL – low density lipoprotein cholesterol, Seco – Secoisolariciresinol, SDG - Secoisolariciresinol diglucoside
In the Canadian study of secoisolariciresinol supplementation (approximately 187 secoisolariciresinol as 543 mg secoisolariciresinol diglucoside per day) and a walking program of ninety-two healthy middle aged adults for twenty-six weeks, HDL, LDL, total cholesterol, triglycerides and the metabolic syndrome composite score were not significantly affected by lignan supplementation.132 However, the administration of secoisolariciresinol diglucoside 600 mg per day (approximately 206 mg secoisolariciresinol) in a Chinese randomized double blind placebo controlled trial136 of fifty-five hypercholesterolemic adults significantly reduced total and LDL over eight weeks. Triglycerides were reduced but not significantly and HDL was not affected. When eleven perimenopausal United States (US) women with mild hyperlipidemia took 200 mg secoisolariciresinol diglucoside per day (approximately 69 mg secoisolariciresinol) for fourteen weeks, LDL, total cholesterol and lipoprotein (a) were reduced.137 When all these data132,133,135-137 were pooled in a meta-analysis,131 total and LDL were significantly reduced, although type of intervention, sex and initial lipid values affected the observed associations. The authors concluded that the association of flaxseed or lignan interventions on blood lipids subjects was suggestive but remained unclear and required further evaluation.131
Other cardiovascular risk factors
As shown in Table 5,132,137,139-141 In a randomized placebo controlled Japanese trial,138 65 mg per day of sesamin significantly reduced total and LDL and apolipoprotein B in twelve hypercholesterolemic adults over eight weeks although HDL and triglycerides were not affected. However, Wu et al's Australian study139 of thirty-three overweight adults using ∼50 mg per day sesamin (39.5 mg sesamin and 12.2 mg sesamolin in 26.2 g sesame seeds) for five weeks had no association with any lipids or C-reactive protein but did lower F2-isoprostanes. It is possible that doses were too low, the study duration was too short, or the other compounds in the sesame seeds such as fatty acids obscured potential positive associations.
Table 5
| Outcome | Author, year, reference | Adult population | Vehicle | Lignan dosage per day | Study duration weeks | N | Response |
|---|---|---|---|---|---|---|---|
| C-reactive protein | Pan et al (2009)141 | M, F, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 64 | -0.45 mg/l p = 0.021 |
| Pan et al (2009)141 | F, China, type 2 diabetics, age 50-70, postmenopausal, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 39 | -0.67 mg/l p = 0.016 | |
| Pan et al (2009)141 | M, China, type 2 diabetics, age 50-70, LDL ≥2.90 mmol/L | Capsule flaxseed lignan extract | Seco 124 mg (360 mg SDG) | 12 | 25 | -0.20 mg/l p = 0.49 | |
| Hallund et al (2008)140 | F, Denmark, postmenopausal age 61 ± 7, healthy, BP<160/90 | Muffin with flaxseed lignan complex | Seco 172 mg (500 mg SDG) | 6 | 22 | -0.18 mg/l p = 0.028 | |
| Marblestone 2008137 | F, USA, perimenopausal, age 36-48 | Flaxseed source by Brevail | Seco 69 mg (200 mg SDG) | 14 | 11 | reduced | |
| Wu et al (2009b)139 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome* or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | -0.11mg/l p = 0.845 | |
| F2-isoprostanes | Wu et al (2009b)139 | M, F, Australia, overweight (BMI 25-35), ≥ 1 risk factor metabolic syndrome or LDL > 3.4 mmol/L | Bars with 26.2 g sesame seeds | Sesamin 39.5, sesamolin 12.2 | 5 | 33 | -35 pmol/l p = 0.047 |
| Metabolic syndrome composite scored | Cornish et al (2009)132 | M Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 39 | 0.34 (controls 0.81, p = 0.058) |
| Cornish et al (2009)132 | F Canada, ≥50 years, healthy, with walking intervention | Tablet flaxseed lignan complex | Seco 187 mg (543 mg SDG) | 26 | 53 | No effect |
Abbreviations: M – males, F – females, BMI – body mass index, BP – blood pressure, LDL – low density lipoprotein cholesterol, Seco – Secoisolariciresinol, SDG - Secoisolariciresinol diglucoside
Five hundred mg secoisolariciresinol diglucoside (172 mg secoisolariciresinol) per day for six weeks blunted a rise in C-reactive protein in twenty-two Danish women, which was evident in controls.140 Pan et al141 found that 360 mg secoisolariciresinol diglucoside (124 mg secoisolariciresinol) per day for twelve weeks significantly decreased C-reactive protein in sixty-four type 2 diabetic patients, particularly women (n=39). Marblestone137 found 69 mg secoisolariciresinol per day for fourteen weeks reduced C-reactive protein in eleven perimenopausal women with mild hyperlipidemia.
Overall it appears that, in sufficient doses, secoisolariciresinol and sesamin may reduce risk factors of cardiovascular disease. However, the studies to date have been mostly small and in populations with varying susceptibility. Clinical trials with flaxseed and sesame seed products have resulted in ambiguous results because little attention was paid to providing an adequate description of the test material. Without knowledge of the actual lignan content in the tested material it is difficult to ascribe outcomes to lignan administration. A major issue with many of the clinical trials (until more recently) is product quality and lack of a detailed description for the tested material. Future controlled trials should focus on target groups at high risk of cardiovascular disease. Interventions should include doses of well-characterized supplement products high enough in lignan content and have trial durations that are long enough to be expected to demonstrate beneficial associations.
Observational studies: Lignan intake
There are significant challenges in measuring lignan intakes, including the incompleteness of food tables, the failure to measure all the lignans present, the inability to account for individual differences in production of enterolignans in the gut, and the failure to use validated biomarkers of intake. Table 6 shows that the evidence for a cardiovascular benefit from dietary lignan intake in existing epidemiological studies is mixed.66,72,75,76,142-145 We found only two studies on dietary intake of lignans and their associations with cardiovascular disease or coronary heart disease events or mortality and five studies with heart disease risk factor endpoints. Milder et al76 assessed lignan intakes in Dutch elderly men and followed them for cardiovascular disease mortality over 15 years. The rate ratios [and 95% confidence interval (CI)] per 1-SD (standard deviation) difference in matairesinol intake (which is metabolized directly to enterolactone) were 0.72 (0.53, 0.98) for coronary heart disease mortality and 0.83 (0.69, 1.00) for cardiovascular disease mortality. Neither total lignans nor the other lignans consumed (lariciresinol, pinoresinol, secoisolariciresinol) were related to coronary heart disease or cardiovascular disease mortality. There was also no association between lignan intakes and diastolic blood pressure, systolic blood pressure, HDL and total cholesterol.
Table 6
| Outcome | Author, year, reference | Study | Population characteristics | Cases (N) | p ≤0.05 | p >0.05 – 0.15 | p > 0.15 |
|---|---|---|---|---|---|---|---|
| Cardiovascular disease mortality b | Milder et al (2006)76 | Prospective cohort, Zutphen, Netherlands | 570 M, elderly, 15 yr follow up | 84 | Mat onlya 0.83 RR (0.69, 1.00) CI p=0.05 [7 μg per SD unit]d |
Seco only 0.88 RR (0.71, 1.08) p = 0.23 [51 μg per SD unit]d |
|
| Coronary heart disease mortality b | Milder et al (2006)76 | Prospective cohort, Zutphen, Netherlands | M, elderly, 15 yr follow up | 570 | Mat only 0.72 RR (0.53, 0.98) CI p=0.03 [7 μg per SD unit]d |
Seco only 0.84 RR (0.61, 1.17) p = 0.31 [51 μg per SD unit]d |
|
| Cardiovascular disease incidence | Van der Schouw et al (2005)66 | Prospective, EPIC, Netherlands | 16,165 F, age 49-70 healthy, 6.25 yr follow up | 518 | 0.89 HR (0.66, 1.19) CI NS [1.39 vs 0.74 mg/d]e,f |
||
| Coronary heart disease Incidence | Van der Schouw et al (2005)66 | Prospective, EPIC, Netherlands | 16,165 F, age 49-70 healthy, 6.25 yr follow up | 371 | 0.92 HR (0.65, 1.29) CI NS [1.39 vs 0.74 mg/d]e,f |
||
| Van der Schouw et al (2005)66 | Prospective, EPIC, Netherlands | 16,165 F, age 49-70 healthy, 6.25 yr follow up sub analysis by smoking status |
n/a | 0.63 HR (0.41, 0.98) CI p for interaction = 0.01 [1.39 vs 0.74 mg/d]e,f smokers |
No association for non-smokers | ||
| Cerebrovascular disease incidence | Van der Schouw et al (2005)66 | Prospective, EPIC, Netherlands | 16,165 F, age 49-70 healthy, 6.25 yr follow up | 147 | 0.80 HR (0.45, 1.42) CI NS [1.39 vs 0.74 mg/d]e,f |
||
| Hypertension | Kreijkamp-Kaspers et al (2004)142 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | 0.49 OR (0.18, 1.29) CI p=0.15 [2.01 vs 1.14 mg/d]d,f |
||
| Blood pressure, diastolic | Kreijkamp-Kaspers et al (2004)142 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | -5.19 mm Hg (-11.41, 1.03) CI p=0.07 [2.01 vs 1.14 mg/d]d,f |
||
| De Kleijn et al (2002)72 | Cross-sectional, Framingham, USA | F, postmenopausal | 939 | -1.1 mg Hg (-3.2, 1.0) CI p for trend = 0.24 [0.79 vs 0.41 mg/d]e,f |
|||
| b | Milder et al (2006)76 | Prospective cohort, Zutphen, Netherlands | M, elderly, 15 yr follow up | 570 | No difference across tertiles of consumption p = 0.77 |
||
| Blood pressure, systolic | Kreijkamp-Kaspers et al (2004)142 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | -7.92 OR (-17.91, 2.07) CI p=0.12 [2.01 vs 1.14 mg/d]d,f |
||
| De Kleijn et al (2002)72 | Cross-sectional, Framingham, USA | F, postmenopausal | 939 | -2.0 mmHg (-5.8, 1.9) CI p for trend = 0.59 [0.79 vs 0.41 mg/d]e,f |
|||
| b | Milder et al (2006)76 | Prospective cohort, Zutphen, Netherlands | M, elderly, 15 yr follow up | 570 | No difference across tertiles of consumption p = 0.47 |
||
| Cholesterol, HDL | De Kleijn et al (2002)72 | Cross-sectional, Framingham, USA | F, postmenopausal | 939 | -2.7 mg/dl (-0.39, 5.02) CI p=0.15 [0.79 vs 0.41 mg/d]e,f |
||
| Kreijkamp-Kaspers et al (2005)143 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | 0.39 mg/dl (-8.8, 6.6) CI p for trend = 0.76 [2.01 vs 1.14 mg/d]d,f |
|||
| b | Milder et al (2006)76 | Prospective cohort, Zutphen, Netherlands | M, elderly, 15 yr follow up | 570 | No difference across tertiles of consumption p = 0.26 |
||
| Cholesterol, LDL | Van der Schouw et al (2005)144 | Cross-sectional, Health Professionals, USA | M, age 47-83 | 301 | 12.9 mg/dl (1.7, 24.1) CI p for trend = 0.01 [1.15 vs 0.39 mg/d medians]e,f |
||
| De Kleijn et al (2002)72 | Cross-sectional, Framingham, USA | F, postmenopausal | 939 | -0.37 mg/dl (-7.3, 6.6) CI p for trend = 0.84 [0.79 vs 0.41 mg/d]e,f |
|||
| Kreijkamp-Kaspers et al (2005)143 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | -8.11 mg/dl (-25.1, 8.9) CI p for trend = 0.35 [2.01 vs 1.14 mg/d]d,f |
|||
| Cholesterol, total | Van der Schouw et al (2005)144 | Cross-sectional, Health Professionals, USA | M, age 47-83 | 468 | 11.1 mg/dl (-2.4, 24.5) CI p for trend = 0.08 [1.15 vs 0.39 mg/d medians]e,f |
||
| De Kleijn et al (2002)72 | Cross-sectional, Framingham, USA | F, postmenopausal | 939 | -2.32 mg/dl (-9.6, 5.0) CI p for trend = 0.47 [0.79 vs 0.41 mg/d]e,f |
|||
| Kreijkamp-Kaspers et al (2005)143 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | -8.11 mg/dl (-26.6, 10.4) CI p for trend = 0.40 [2.01 vs 1.14 mg/d]d,f |
|||
| b | Milder et al (2006)76 | Prospective cohort, Zutphen, Netherlands | M, elderly, 15 yr follow up | 570 | No difference across tertiles of consumption p = 0.52 |
||
| Triglycerides | De Kleijn et al (2002)72 | Cross-sectional, Framingham, USA | F, postmenopausal | 939 | -20.4 mg/dl (-32.7, -7.96) CI p=0.001 [0.79 vs 0.41 mg/d]e,f |
||
| Kreijkamp-Kaspers et al (2005)143 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | -2.65 mg/dl (-12.4, 11.5) CI p for trend = 0.78 [2.01 vs 1.14 mg/d]d,f |
|||
| Apolipoprotein B | Van der Schouw et al (2005)144 | Cross-sectional, Health Professionals, USA | M, age 47-83 | 468 | 10.0 mg/dl (1.6, 18.4) CI p for trend = 0.02 [1.15 vs 0.39 mg/d medians]e,f |
||
| Lipoprotein (a) | Kreijkamp-Kaspers et al (2005)143 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | 0.47 OR (0.17, 1.31) CI p for trend = 0.18 [2.01 vs 1.14 mg/d]d,f |
||
| C-peptide | Van der Schouw et al (2005)144 | Cross-sectional, Health Professionals, USA | M, age 47-83 | 468 | -0.55 ng/dl (-0.97, -0.13) CI p for trend = 0.01 [1.15 vs 0.39 mg/d medians]e,f |
||
| Ankle brachial index | Kreijkamp-Kaspers et al (2004)142 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | 0.01 (-0.04, 0.07 p for trend = 0.60 [2.01 vs 1.14 mg/d]d,f |
||
| Endothelial function (pimax) | Kreijkamp-Kaspers et al (2004)142 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | -0.01 pimax (-0.08, 0.06 p for trend = 0.80 [2.01 vs 1.14 mg/d]d,f |
||
| Flow mediated dilatationc | Pellegrini et al (2010)75 | Cross-sectional, longitudinal follow-up Italy | 242 M, F, healthy, postmenopausal | 101 | Mat only 4.1% to 8.1% change p for trend = 0.016 [0.039 vs 0.009 mg/d means]e |
Seco only 4.6% to 6.8% change p for trend = 0.099 [0.625 vs 0.158 mg/d means]e |
|
| Kreijkamp-Kaspers et al (2004)142 | Cross-sectional, EPIC, Netherlands | F, postmenopausal, age 60-75, healthy | 301 | -0.09 % (-1.93, 2.12) CI p for trend = 0.92 [2.01 vs 1.14 mg/d]d,f |
|||
| Reduced aortic stiffness | Van der Schouw et al (2002)145 | Cross-sectional, EPIC, Netherlands | F, healthy, postmenopausal | 403 | -0.41 (-0.93, 0.11) CI p for trend = 0.06 [0.87 vs 0.33 mg/d]e,f |
||
| Van der Schouw et al (2002)145 | Cross-sectional, EPIC, Netherlands | F, healthy, long (20-30 years) postmenopausal time span | 180 | -0.80 (-1.54, -0.05) CI p for trend = 0.03 [0.87 vs 0.33 mg/d]e,f |
|||
| Van der Schouw et al (2002)145 | Cross-sectional, EPIC, Netherlands | F, healthy, short (8-12 years) postmenopausal time span | 199 | -0.19 (-0.95, 0.57) CI p for trend = 0.40 [0.87 vs 0.33 mg/d]e,f |
|||
| Metabolic syndrome score | De Kleijn et al (2002)72 | Cross-sectional, Framingham, USA | F, postmenopausal | 939 | -0.55 (-0.82, -0.28) CI p=0.0001 [0.79 vs 0.41 mg/d]e,f |
||
| Waist hip ratio | De Kleijn et al (2002)72 | Cross-sectional, Framingham, USA | F, postmenopausal | 939 | -0.017 (-0.030, -0.0016) CI p=0.03 [0.79 vs 0.41 mg/d]e,f |
Abbreviations: M – males, F – females, CI – 95% Confidence interval, HR – Hazard rate ratio, OR – Odds ratio, RR – Rate ratio, [ ] – definition of comparison groups or per SD unit, Seco – Secoisolariciresinol, Mat – matairesinol
Underlying causes of death coded according to the International Classification of Diseases, 9th and 10th revisions (ICD-9 and ICD-10). Cardiovascular disease deaths defined as ICD-9 codes 390-459 and ICD-10 codes I20-I99, coronary heart disease deaths as ICD-9 codes 410-414 (ischemic heart disease) and 492.2 (atherosclerotic heart disease) and ICD-10 codes I20-I25, and stroke as ICD-9 codes 430-438 (ischemic and hemorrhagic cerebrovascular disease) and ICD-10 codes I60-I69 (cerebrovascular diseases).
In the Dutch EPIC cohort study66 of women who were followed for a median of 6.25 years, lignan intakes (matairesinol and secoisolariciresinol) of approximately 1100 μg/day were not associated with CVD disease risk. However, increasing lignan intake was associated with lower CHD risk but only among smokers. Relationships with individual lignans were not reported in that study.66
Supporting Milder et al's findings,76 Pellegrini et al75 also found greater matairesinol intakes were significantly associated with increased flow mediated dilatation in older Italian men and women but no significant associations were observed with secoisolariciresinol, pinoresinol, or lariciresinol or total lignans. These findings are intriguing because, compared to the other lignans, matairesinol is found in much lower amounts (e.g., only a tenth) than other lignans in the diet.
Three observational studies examining blood pressure outcomes found negligible associations with greater lignan intake.72,76,142 In a cross-sectional study of postmenopausal women in the US, lignan intake was associated with a borderline, non-statistically significant association with lower diastolic or systolic blood pressure.72 In a Dutch cross-sectional study of women, although there was a trend toward lower systolic and diastolic blood pressure and a lesser prevalence of hypertension with higher intake (albeit levels of 750 μg) of two lignans (matairesinol and secoisolariciresinol), these findings were not significant.142
Observational studies of the relationship between lignan intake and total cholesterol and its subfractions are mixed. Two observational studies72,143 of Dutch and US women had no significant association with LDL, HDL or total cholesterol, but a third observational study144 of US men found that lignan intake was associated significantly with increased LDL and apolipoprotein B and nonsignificantly with increased total cholesterol. This same study found that lignan intake was significantly associated with lower C-peptide.144 In the cross-sectional study in Framingham, Massachusetts (USA), postmenopausal women with greater intake of lignans had a lower fasting triglyceride concentration.72
In regard to markers of vascular function, in addition to the aforementioned study of flow mediated dilatation,75 a Dutch study145 found lignan intake non-significantly associated with reduced aortic stiffness in all postmenopausal women but significantly associated with reduced aortic stiffness in the subset of women 20 to 30 years beyond menopause. Kreijkamp-Kasper et al's study142 also examined these markers in Dutch postmenopausal women but found no significant association with endothelial function, flow mediated dilatation, and ankle brachial index.
In the Milder et al prospective cohort76 and the Pellegrini et al cross-sectional vascular75 studies, which measured specific lignan dietary intakes, matairesinol appeared to be the lignan most commonly associated with decreased cardiovascular disease risk. However, this may have been simply because matairesinol is more commonly measured in foods compared to the other lignans. Matairesinol occurs particularly in wine, oats and rye. Among populations consuming wine, the amount of matairesinol provided from this source could be high enough (e.g., 17-22 mg/100g white wine or 74-98 mg/100g red wine) to provide additional protection beyond the alcohol content alone, although confounding by other components of wine cannot be ruled out. Many epidemiologic studies146 show that whole grain intake is cardioprotective. Matairesinol may be one of the important components responsible for this association. The soluble fiber content of oats is known to be cardioprotective, and the considerable matairesinol content of whole grain oats may contribute to these protective associations.
Observational studies: Serum enterolactone
Serum levels of enterolactone are somewhat better measures of systemic lignan exposure in tissues than are lignan intakes alone, although the short half-life of both metabolites requires caution in interpreting studies of a single measure of either biomarker in relation to disease outcome.60,78 It may be useful to keep in mind that it has been suggested that levels of enterolactone above 30 nmol/l or higher are protective, and levels below 15 nmol/l are too low to confer protections. Upper levels of enterolactone from diet appear to be 90-100 nmol/l, however variations in levels are high.147,148 Table 7 illustrates findings from four epidemiological studies that examined plasma enterolactone in relation to risk of coronary heart disease mortality, cardiovascular disease mortality and events, and other related risk factors (blood pressure, HDL and LDL).149-153 In most of the studies in Table 7, the highest quartiles and quintiles approached or were in the putative protective range.
Table 7
| Outcome | Author, year, reference | Study | Population characteristics | Cases (N) | p ≤0.05 | p >0.05 – 0.15 | p > 0.15 |
|---|---|---|---|---|---|---|---|
| Cardiovascular disease mortality | Vanharanta et al (2003)149 | Prospective, Kuopio, Finland | 1889 M, age 42, 48, 54, or 60, 12.2 yr follow up | 103 | 0.55 RR (0.29, 1.01) CI p=0.04 [23.9 vs 6.9 nmol/l]a |
||
| Coronary heart disease mortality | Vanharanta et al (2003)149 | Prospective, Kuopio, Finland | M, age 42, 48, 54, or 60, 12.2 yr follow up | 70 | 0.44 RR (0.20, 0.96) CI p=0.03 [23.9 vs 6.9 nmol/l]a |
||
| Kilkkinen et al (2006)151 | Prospective case-cohort, ATBC, Finland | M, smokers, 11.1 yr follow up | 340 cases (205 MI, 135 deaths) 420 controls | 0.57 RR (0.26, 1.25) CI p for trend=0.18 [28.25 vs 5.02 nmol/l]b |
|||
| Coronary heart disease risk (acute events & mortality) | Kilkkinen et al (2006)151 | Prospective case-cohort, ATBC, Finland | M, smokers, 11.1 yr follow up | 340 cases (205 MI, 135 deaths) 420 controls | 0.63 RR (0.33, 1.11) CI p for trend=0.07 [28.25 vs 5.02 nmol/l]b |
||
| Coronary heart disease risk (acute events) | Kilkkinen et al (2006)151 | Prospective case-cohort, ATBC, Finland | M, smokers, 11.1 yr follow up | 340 cases (205 MI, 135 deaths) 420 controls | 0.67 RR (0.37, 1.23) CI p for trend=0.10 [28.25 vs 5.02 nmol/l]b |
||
| c | Kuijsten et al (2009)152 | Prospective nested case control Netherlands | M, F, age 20-59, 11 yr follow up | 236 cases, 283 controls | 1.51 OR (0.87, 2.61) CI p for trend = 0.12 [17.5 vs 3.8 nmol/l]a |
||
| c | Kuijsten et al (2009)152 | Prospective nested case control Netherlands | F, premenopausal, age 20-59, 11 yr follow up | 34 cases | 0.16 OR (0.02, 1.03) CI p for trend = 0.05 [per 13.7 nmol/l, 17.5 vs 3.8 nmol/l]a |
||
| c | Kuijsten et al (2009)152 | Prospective nested case control Netherlands | F, postmenopausal, age 20-59, 11 yr follow up | 30 cases | 1.17 OR END (1.00, 1.36) CI p for trend = 0.05 [per 1.3 nmol/l, 1.7 vs 0.4 nmol/l]a |
2.27 OR (0.57, 8.95) CI p for trend = 0.24 [per 13.7 nmol/l, 17.5 vs 3.8 nmol/l]a |
|
| Vanharanta et al (1999)150 | Prospective nested case control, Kuopio, Finland | M, age 42, 48, 54, or 60, 7.7 yr follow up | 167 cases, 167 controls | 0.35 OR (0.14, 0.88) CI p=0.03 [30.1 vs 7.2 nmol/l]a |
|||
| Hypertension | Vanharanta et al (2003)149 | Prospective, Kuopio, Finland | M, age 42, 48, 54, or 60, 12.2 yr follow up | 1889 at baseline | -12 % p for heterogeneity <001 [23.9 vs 6.9 nmol/l]a |
||
| Blood pressure, diastolic | Vanharanta et al (1999)150 | Prospective nested case control, Kuopio, Finland | M, age 42, 48, 54, or 60, 7.7 yr follow up | 167 cases, 167 controls at baseline | -3 mm Hg p for heterogeneity =0.017 [30.1 vs 7.2 nmol/l]a |
||
| Vanharanta et al (2003)149 | Prospective, Kuopio, Finland | M, age 42, 48, 54, or 60, 12.2 yr follow up | 1889 at baseline | -3 mm Hg p for heterogeneity <001 [23.9 vs 6.9 nmol/l]a |
|||
| c | Kuijsten et al (2009)152 | Prospective nested case control Netherlands | M, F, age 20-59, 11 yr follow up | 283 controls at baseline | -3 mm Hg p for trend = 0.08 [17.5 vs 3.8 nmol/l]a |
||
| Kilkkinen et al (2006)151 | Prospective case-cohort, ATBC, Finland | M, smokers, 11.1 yr follow up | 420 controls at baseline | -6 mm Hg p for heterogeneity = 0.21 [28.25 vs 5.02 nmol/l]b |
|||
| Blood pressure, systolic | Vanharanta et al (2003)149 | Prospective, Kuopio, Finland | M, age 42, 48, 54, or 60, 12.2 yr follow up | 1889 at baseline | -5 mm Hg p for heterogeneity <001 [23.9 vs 6.9 nmol/l]a |
||
| Vanharanta et al (1999)150 | Prospective nested case control, Kuopio, Finland | M, age 42, 48, 54, or 60, 7.7 yr follow up | 167 cases, 167 controls at baseline | -5 mm Hg p for heterogeneity =0.026 [30.1 vs 7.2 nmol/l]a |
|||
| c | Kuijsten et al (2009)152 | Prospective nested case control Netherlands | M, F, age 20-59, 11 yr follow up | 283 controls at baseline | -4 mm Hg p for trend = 0.09 [17.5 vs 3.8 nmol/l]a |
||
| Kilkkinen et al (2006)151 | Prospective case-cohort, ATBC, Finland | M, smokers, 11.1 yr follow up | 420 controls at baseline | -6 mm Hg p for heterogeneity = 0.84 [28.25 vs 5.02 nmol/l]b |
|||
| Cholesterol, HDL | Kuijstenc et al (2009)152 | Prospective nested case control Netherlands | M, F, age 20-59, 11 yr follow up | 283 controls at baseline | 3.86 mg/dl p for trend = 0.07 [17.5 vs 3.8 nmol/l]a |
||
| Vanharanta et al (1999)150 | Prospective nested case control, Kuopio, Finland | M, age 42, 48, 54, or 60, 7.7 yr follow up | 167 cases, 167 controls at baseline | 1.54 mg/dl p for heterogeneity = 0.74 [30.1 vs 7.2 nmol/l]a |
|||
| Vanharanta et al (2003)149 | Prospective, Kuopio, Finland | M, age 42, 48, 54, or 60, 12.2 yr follow up | 1889 at baseline | 1 mg/dl p for heterogeneity = 0.66 [23.9 vs 6.9 nmol/l]a |
|||
| Kilkkinen et al (2006)151 | Prospective case-cohort, ATBC, Finland | M, smokers, 11.1 yr follow up | 420 controls at baseline | 1.16 mg/dl p for heterogeneity = 0.47 [28.25 vs 5.02 nmol/l]b |
|||
| Cholesterol, LDL | Vanharanta et al (1999)150 | Prospective nested case control, Kuopio, Finland | M, age 42, 48, 54, or 60, 7.7 yr follow up | 167 cases, 167 controls at baseline | -2.32 mg/dl p for heterogeneity = 0.52 [30.1 vs 7.2 nmol/l]a |
||
| Vanharanta et al (2003)149 | Prospective, Kuopio, Finland | M, age 42, 48, 54, or 60, 12.2 yr follow up | 1889 at baseline | 0.0 mg/dl p for heterogeneity = 0.91 [23.9 vs 6.9 nmol/l]a |
|||
| Cholesterol, total | Kuijsten c et al (2009)152 | Prospective nested case control Netherlands | M, F, age 20-59, 11 yr follow up | 283 controls at baseline | 5.80 mg/dl p for trend = 0.22 [17.5 vs 3.8 nmol/l]a |
||
| Vanharanta et al (1999)150 | Prospective nested case control, Kuopio, Finland | M, age 42, 48, 54, or 60, 7.7 yr follow up | 167 cases, 167 controls at baseline | 3.47 mg/dl p for heterogeneity = 0.82 [30.1 vs 7.2 nmol/l]a |
|||
| Kilkkinen et al (2006)151 | Prospective case-cohort, ATBC, Finland | M, smokers, 11.1 yr follow up | 420 controls at baseline | 3.86 mg/dl p for heterogeneity = 0.22 [28.25 vs 5.02 nmol/l]b |
|||
| Apolipoprotein B | Vanharanta et al (1999)150 | Prospective nested case control, Kuopio, Finland | M, age 42, 48, 54, or 60, 7.7 yr follow up | 167 cases, 167 controls at baseline | 41 mg/l p for heterogeneity = 0.22 [30.1 vs 7.2 nmol/l]a |
||
| Reduced F2-isoprostanes | Vanharanta et al (2002)153 | Cross-sectional, ASAP, Finland | M, age 58.6 ± 6.5 | 100 | -37.4% p for trend = 0.008 [25.6 vs 3.9 nmol/l]b |
Abbreviations: M – males, F – females, CI – 95% Confidence Interval, OR – Odds ratio, RR – Rate ratio, [ ] – definition of comparison groups or per SD (standard deviation) unit, END – enterodiol, MI – myocardial infarction
Underlying causes of death coded according to the International Classification of Diseases, 9th and 10th revisions (ICD-9 and ICD-10). Cardiovascular disease deaths defined as ICD-9 codes 390-459 and ICD-10 codes I20-I99, coronary heart disease deaths as ICD-9 codes 410-414 (ischemic heart disease) and 492.2 (atherosclerotic heart disease) and ICD-10 codes I20-I25, and stroke as ICD-9 codes 430-438 (ischemic and hemorrhagic cerebrovascular disease) and ICD-10 codes I60-I69 (cerebrovascular diseases).
In a cohort study of 1889 Finnish men who were followed for an average of 12.2 years, Vanharanta et al149 found lower risk of fatal coronary heart disease [Rate Ratio (RR) = 0.44, p=0.03] and fatal cardiovascular disease (RR 0.55, p=0.04) with greater concentrations of serum enterolactone. In contrast, associations with all-cause mortality were weaker and not significant (data not shown).149
In a nested case-control study of healthy Finnish men where blood levels of enterolactone was measured up to 7.7 years prior to diagnosis, those with mean serum concentrations of enterolactone in the highest quartile (>30.1 nmol/L) had a 65.3% lower risk of acute coronary events than men in lowest quartile (<7.21 nmol/L).150 In another Finnish study among men, where blood was measured up to 11 years before diagnosis, the association between mean serum enterolactone concentration and coronary heart disease risk (acute and fatal) was not significant in cases compared to healthy controls (17.8 nmol/L vs 18.1 nmol/L) when adjusted for classic risk factors.151 In a Dutch nested case control study of men and women, no significant differences were found between serum enterolactone levels in coronary heart disease acute events.152
Two Finnish nested case-control studies149,150 of blood pressure noted significant inverse associations with plasma enterolactone, where as in a third Finnish study151 and a Dutch study152 associations were not statistically significant. All of the nested case control studies of cardiovascular disease and coronary heart disease outcomes found no association between enterolactone levels and blood lipids (HDL, LDL, total cholesterol, apolipoprotein B), apart from a borderline positive association in one Dutch study.152 In a cross-sectional Finnish study, high enterolactone levels were associated with reduced F2-isoprostanes, a marker of lipid peroxidation.153 The weak correlations between serum enterolactone and cardiovascular disease outcomes make it difficult to draw conclusions from those studies.
Limitations of existing studies
Although many of the studies reviewed suggest possible associations with dietary or biomarker measures of lignan exposure, several limitations are worth noting. More research on the food content of lignans, and on food sources in relation to health outcomes in epidemiologic studies is needed. It may be that a certain threshold of intake is required and many Western populations either do not reach those levels, or the appropriate foods are not assessed on research questionnaires. If possible, repeated measures of these biomarkers would benefit studies of the association between enterolactone and chronic disease outcomes. Finally, it is of interest that most studies of the lignan intake were of women, whereas all but one of the enterolactone studies were of men. Because associations with lignans may vary by sex, more research including both men and women is needed. Future studies should employ both complete dietary intakes of lignans and serum (or plasma) enterolignan markers in high-risk groups.
Are lignans the components that provide cardiovascular benefits?
Can the cardioprotective benefits of foods rich in lignans be ascribed to lignans, lignins, dietary fiber, alkylresorcinols or other components? Both lignins and lignans are synthesized from similar subunits, and both, as well as other components of dietary fiber, are commonly found in cereals and grains.
Of the few studies on lignins, the various types of lignins in foods were not examined independently. One study of fiber components154 and a recent review155 found that lignin did not lower lipids. However, several observational studies examining lignin and cancer risk found reduced risk of colorectal156,157 oral, pharyngeal and esophageal158 cancers but not with breast,159 ovarian,160 and renal161 cancers.
Dietary fiber may be responsible in part for associations observed with lignan intake. Dietary fiber,162-166 particularly soluble fiber,164,167-169 reduces risk of cardiovascular disease. Dietary fiber lowers blood pressure,170,171 decreases C-reactive protein levels,172-176 decreases metabolic syndrome177-179 and decreases insulin resistance.180 Although some studies indicate dietary fiber has weak lipid lowering associations,181,182 soluble fiber is more highly associated with lower serum lipids,183-188 lower blood pressure189 and fewer symptoms of metabolic syndrome.190,191
Cereal fiber consists more of insoluble fibers (lignins) than soluble fibers. Cereal fiber is associated with decreased insulin resistance,192 lower serum lipids,193 lower blood pressure,194,195 less progression of coronary atherosclerosis in postmenopausal women with established coronary artery disease,196 reduced risk not only of coronary heart disease,165,197,198 but also of cardiovascular disease199 and stroke200 in many201 but not all studies.164,202,203 Insoluble fiber is associated with lower blood pressure,189 lower C-reactive protein levels,176 lower insulin resistance,180 and lower risk of both cardiovascular disease168 and myocardial infarction.164,168
The studies on lignan intakes and cardiovascular disease risk, which were reviewed earlier in the article,66,72,75,76,142-145 were adjusted for fiber intakes, but not separately for insoluble and soluble fiber. Thus, the associations with lignan intake described here were over and above those of fiber. In the four epidemiological studies using enterolactone as the marker of lignan intakes, results were mixed.149-152 Serum enterolactone was positively correlated with fiber intake in one study150 but fiber intake had no consistent association with the risk of acute coronary events. In a second study by the same investigator149 energy adjusted fiber intake was associated with enterolactone and explained 6% of its variation. However, in a third study, another group of investigators151 found that adjusting for fiber and other dietary factors had little association with results. The fourth study also found fiber intake significantly associated with plasma enterolactone and enterodiol.152 It is possible that enterolactone is a biomarker for a heart-healthy diet, and that such a diet exerts its effects through many different constituents (alkylresorcinols, flavonoids, glucosinolates, lignans, lignins, phenolic acids, stilbenes, terpenes, etc.).
In cereals the fiber fraction contains alkylresorcinols, folic acid, polyphenols, Vitamin E and other factors in addition to lignans, which may also be involved in cardioprotection. However, in some cohort studies the associations between lignan intake and cardiovascular disease mortality remain even after adjusting for dietary fiber intakes. Whether the associations observed with coronary heart disease and cardiovascular disease risk and lignan exposure might be explained by intakes of cereal fiber or alcohol rather than by lignan intakes themselves remains to be determined. Nevertheless, in several controlled trials that used higher lignan doses than usually found in diets, such as a secoisolariciresinol diglucoside enriched source (500 mg/day secoisolariciresinol diglucoside), there were positive associations. Since secoisolariciresinol diglucoside enriched products are available today and some cardiovascular risk reducing associations were noted with their use, there is some support for a role of lignans in cardiovascular disease risk reduction. Now that high quality products with well-characterized lignan contents are available, studies done with these well-characterized products may shed light on whether lignans do in fact have cardioprotective properties. Studies in experimental animals will also be helpful, particularly in exploring possible mechanisms of action.
Conclusion
There is intriguing but not yet compelling evidence from epidemiological studies that lignans present in the very small quantities typical of usual Western diets, decrease coronary heart disease and cardiovascular disease mortality. More research is needed to confirm or refute these associations. Intervention studies using higher doses have found positive associations with some cardiovascular risk factors. In addition, it is important to elucidate whether doses found in foods or only the larger doses that might be delivered in dietary supplements offer protection.
Acknowledgments
Funding and support. This work was supported in part with resources from the United States Department of Agriculture (USDA), Cooperative State Research, Education, and Extension Service grant #2006-35200-17259, the USDA, Agricultural Research Service, under agreement No. 58-1950-7-707, and NIH National Heart, Lung and Blood Institute grant R21HL087217. Any opinions, findings, conclusions or recommendations expressed here are those of the authors and do not necessarily reflect the view of the USDA.
Abbreviations
- BMI
- body mass index
- BP
- blood pressure
- CI
- confidence interval
- HDL
- high density lipoprotein cholesterol
- HR
- hazard rate ratio
- LDL
- low density lipoprotein cholesterol
- OR
- odds ratio
- RR
- rate ratio
- SD
- standard deviation
- US or USA
- United States of America
- USDA
- United States Department of Agriculture
Footnotes
Conflict of Interest: None